Your How much does one mole of glucose weigh images are ready. How much does one mole of glucose weigh are a topic that is being searched for and liked by netizens now. You can Get the How much does one mole of glucose weigh files here. Get all royalty-free photos.
If you’re searching for how much does one mole of glucose weigh pictures information linked to the how much does one mole of glucose weigh keyword, you have pay a visit to the ideal blog. Our site always gives you suggestions for viewing the maximum quality video and picture content, please kindly hunt and locate more enlightening video articles and graphics that fit your interests.
How Much Does One Mole Of Glucose Weigh. A mole is 60221407610²³. Molecular weight of Glucose or grams. Likewise to get a mole of glucose we need 6 moles of carbon 12 moles of hydrogen and 6 moles of oxygen. 1 mole is equal to 1.
 How Many Number Of Moles Of Glucose Are Present In 540 Grams Of Glucose Calculate The Weight Nf 0 1 Molec Sodium Carbonate From toppr.com
How Many Number Of Moles Of Glucose Are Present In 540 Grams Of Glucose Calculate The Weight Nf 0 1 Molec Sodium Carbonate From toppr.com
Molecular weight of Glucose or grams The molecular formula for Glucose is C6H12O6. Polar and forms hydrogen bonds with itself. CAS Registry Number CAS RN. How To Get Rid Of Dim Spots On Encounter In a single day Brownspots Removebrownspotsonarms Molesappearingonskin Pores and skin Moles Pores and skin Tag Mole Water. What is the molar mass of sugar. 1 mole is equal to 1 moles Glucose or 18015588 grams.
05 moleliter x 1000 mmolesmole 500 mM.
Thus 1 mole of glucose weighs 180 g. All of this weighs. So altogether the molar mass of a single molecule of glucose is equal to. How much does one molecule of fat with the molecular formula C51H98O6 weigh. 1 mol of C4H10 weighs 58 grams and contains 602E23 molecules of C4H10. Molar mass of glucose is 180 gmol.
 Source: dynamicscience.com.au
Source: dynamicscience.com.au
Molar mass of C6H12O6 18015588 gmol. 1201076 10079412 1599946 Percent composition by element. 6 moles Glucose to grams 108093528 grams. Convert grams Glucose to moles or moles Glucose to grams. A mole is 60221407610²³.
 Source: sciencetrends.com
Source: sciencetrends.com
05 moleliter x 180 gramsmole x 1 liter 90 g How many molecules of glucose are in that 1 liter of 05M glucose solution. Thus 1 mole of glucose weighs 180 g. A mole is the quantity of a substance whose weight in grams is equal to the molecular weight of the substance. You can view more details on each measurement unit. Polar and forms hydrogen bonds with itself.
 Source: toppr.com
Source: toppr.com
Glucose weighs 156 gram per cubic centimeter or 1 560. How many moles of glucose are in 300g of glucose. 1 mole is equal to 1 moles Glucose or 18015588 grams. The SI base unit for amount of substance is the mole. Why is water cohesive.
 Source: slideplayer.com
Source: slideplayer.com
They weigh 180 grams per mole. Izzy2162 izzy2162 3423grams per mole gmol Its digestive system detoxifies the eucalyptus oil a poison to other animals. 05 moleliter x 1000 mmolesmole 500 mM. Weight to volume volume to weight price mole to volume and weight mass and molar concentration density About this page. 6 moles Glucose to grams 108093528 grams.
 Source: slideplayer.com
Source: slideplayer.com
So mass of 2 moles of glucose is. Glucose weighs 156 gram per cubic centimeter or 1 560. How much does one molecule of fat with the molecular formula C51H98O6 weigh. 05 moleliter x 1 liter x 6023x1023 moleculesmole 3012x1023 molecules What is the concentration of the 05M glucose solution expressed in mM. How much does one sucrose C12H22O11 molecule weigh in grams.
 Source: youtube.com
Source: youtube.com
Molar mass of C6H12O6 18015588 gmol. 612 121 116 180 grams. We need 2 moles of glucose to prepare 1 litre of 2M solution. How much does 1 sucrose molecule weigh in grams. Likewise to get a mole of glucose we need 6 moles of carbon 12 moles of hydrogen and 6 moles of oxygen.
 Source: clutchprep.com
Source: clutchprep.com
The total number of atoms in 1 mol C4H10 is 14 x 602E23 because each molecule of C4H10 contains four carbon atoms and ten hydrogen atoms as indicated in the formula C4H10. One mole of iron atoms weighs 55845 g or 55845 g mol One mole of H 2 O molecules weighs 180153 g or 180153 g mol One mole of NaCl formula units weighs 58443 g or 58443 g mol In general we can refer to the weight of one mole of a pure substance as its molar mass. 05 moleliter x 180 gramsmole x 1 liter 90 g How many molecules of glucose are in that 1 liter of 05M glucose solution. Polar and forms hydrogen bonds with itself. Molar mass of glucose is 180 gmol.
 Source: slideplayer.com
Source: slideplayer.com
Why is water cohesive. The total number of atoms in 1 mol C4H10 is 14 x 602E23 because each molecule of C4H10 contains four carbon atoms and ten hydrogen atoms as indicated in the formula C4H10. So altogether the molar mass of a single molecule of glucose is equal to. Molar mass of C6H12O6 18015588 gmol. Polar and forms hydrogen bonds with itself.
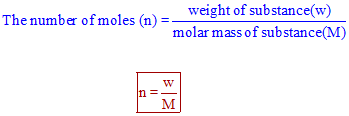 Source: adichemistry.com
Source: adichemistry.com
So altogether the molar mass of a single molecule of glucose is equal to. The SI base unit for amount of substance is the mole. How To Get Rid Of Dim Spots On Encounter In a single day Brownspots Removebrownspotsonarms Molesappearingonskin Pores and skin Moles Pores and skin Tag Mole Water. How many moles of glucose are in 300g of glucose. Molarity Moles of solute Volume of solution in litres 2 M n 1 L.
 Source: sciencetrends.com
Source: sciencetrends.com
The molecular formula for Glucose is C6H12O6. One glucose molecule does not weigh 180 grams. Glucose weighs 156 gram per cubic centimeter or 1 560. 05 moleliter x 1 liter x 6023x1023 moleculesmole 3012x1023 molecules What is the concentration of the 05M glucose solution expressed in mM. In this regard how much does a mole of glucose weigh.

To get a single glucose molecule C6H12O6 we need 6 carbon atoms 12 hydrogen atoms and finally 6 oxygen atoms. 1 mole is equal to 1 moles Glucose or 18015588 grams. Glucose has a molar mass of 18016 gmol. A mole is 60221407610²³. Does a mole of neon atoms weigh the same as a mole of aluminum.

Does a mole of neon atoms weigh the same as a mole of aluminum. 1 mole is equal to 1 moles Glucose or 18015588 grams. 612 121 116 180 grams. 1 mol of C4H10 weighs 58 grams and contains 602E23 molecules of C4H10. How much does one sucrose C12H22O11 molecule weigh in grams.
 Source: chegg.com
Source: chegg.com
If 1 molecule of glucose weighs 180 grams does 6 molecules make 1 kg. We need 2 moles of glucose to prepare 1 litre of 2M solution. The molecular formula for Glucose is C6H12O6. Molarity Moles of solute Volume of solution in litres 2 M n 1 L. In case you weight out precisely 342 grams g of sucrose youll have weighed out 1 moleof it.

34230 gmol or one mole of sugar is 34230 g. So why does one mole of sugar weight more than one mole of water. Molarity Moles of solute Volume of solution in litres 2 M n 1 L. Molecular weight of Glucose or grams The molecular formula for Glucose is C6H12O6. Because an individual molecule of sugar weighs more than an.

612 121 116 180 grams. Ergo 6 molecules of glucose would weigh significantly less than one kilogram. 1 mole is equal to 1 moles Glucose or 18015588 grams. 6 moles Glucose to grams 108093528 grams. 05 moleliter x 1 liter x 6023x1023 moleculesmole 3012x1023 molecules What is the concentration of the 05M glucose solution expressed in mM.
This site is an open community for users to do sharing their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site value, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title how much does one mole of glucose weigh by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






