Your How much does 1 mole of water weigh images are ready. How much does 1 mole of water weigh are a topic that is being searched for and liked by netizens now. You can Download the How much does 1 mole of water weigh files here. Find and Download all royalty-free photos and vectors.
If you’re looking for how much does 1 mole of water weigh images information connected with to the how much does 1 mole of water weigh interest, you have come to the ideal blog. Our website always gives you suggestions for refferencing the maximum quality video and image content, please kindly search and locate more informative video articles and graphics that fit your interests.
How Much Does 1 Mole Of Water Weigh. A person drinks 150 x 103 g of water H2O per day. 18 amu is the mass of one molecule of water. Convert grams to pounds. 1 oz 12 12 oz 1 dozen eggs.
 Chemistry Warm Up Mole Mass Particles 1 What Is The Mass Of One Mole Of Water 2 If One Milliliter Of Water Has A Mass Of 1 00grams How Many Moles Ppt Download From slideplayer.com
Chemistry Warm Up Mole Mass Particles 1 What Is The Mass Of One Mole Of Water 2 If One Milliliter Of Water Has A Mass Of 1 00grams How Many Moles Ppt Download From slideplayer.com
Polar and forms hydrogen bonds with itself. You can view more details on each measurement unit. This assumes a water temperature of 392F 40C. A mole is a convenient counting unit whenever one is dealing with numbers of atoms or molecules. Correspondingly what does 1 mole of water weigh. 18 amu is the mass of one molecule of water.
18 gm per mol is the mass of one mole of water.
A mole is a convenient counting unit whenever one is dealing with numbers of atoms or molecules. One mole of water is about 18 milliliters. 99802 00022 21956. 1000 099802 99802g. Therefore one mole of water weighs 180152 grams. Click to see full answer.
 Source: courses.lumenlearning.com
Source: courses.lumenlearning.com
Approximately how much does one mole of glucose C6H12O6 weigh. You can also use our weight conversion calculators to convert from grams and kilograms to pounds and ounces. 1 000 000 000. Why is water cohesive. Weight of water 2 10079 g 159994 g.
 Source: slideplayer.com
Source: slideplayer.com
This is why one mole of sugar has a. You have a sample of 1204 x 1024 atoms of gold. 18 gm per mol is the mass of one mole of water. You have one mole of water H 2 moles of atom 2g O 1 mole of atom 16g So adding the two we get the weight of one mole of water which is 18g. If I wanted to know how much a dozen eggs weighed I could take the average weight for one egg and multiply by 12.
 Source: pinterest.com
Source: pinterest.com
We assume you are converting between moles Water and gram. How many molecules are present in one mole of water H2O. Weight in grams volume density. Water molecular weight. How much does this sample weigh.
 Source: chemistry.wustl.edu
Source: chemistry.wustl.edu
The molecular formula for Water is H2O. For example the average mass of one molecule of water is about 180153 daltons and one mole of water is about 180153 gramsDalton unit. How many molecules are present in one mole of water H2O. H2O 12 16 18 amu. 0 18 g О 44 g ооо o 759.
 Source: pinterest.com
Source: pinterest.com
If we have one mole of water then we know that it will have a mass of 2 grams for 2 moles of H atoms 16 grams for one mole O atom 18 grams. If we have one mole of water then we know that it will have a mass of 2 grams for 2 moles of H atoms 16 grams for one mole O atom 18 grams. 1 ml x 1gml 1g. The SI base unit for amount of substance is the mole. We assume you are converting between moles Water and gram.
 Source: slideplayer.com
Source: slideplayer.com
There actually are simple It is not exactly equal to but one ml of water has a mass of 1 g because the density of water at 25 C is 1 gml. How much does 1 mole of magnesium weigh in grams. 18 amu is the mass of one molecule of water. We could do the same thing with atoms – for example a mol of helium. You have a sample of 1204 x 1024 atoms of gold.
 Source: ru.pinterest.com
Source: ru.pinterest.com
1 000 000 000 000. We assume you are converting between moles Water and gram. If we have one mole of water then we know that it will have a mass of 2 grams for 2 moles of H atoms 16 grams for one mole O atom 18 grams. What is 1 mole of water equal to. You have one mole of water H 2 moles of atom 2g O 1 mole of atom 16g So adding the two we get the weight of one mole of water which is 18g.
 Source: slideplayer.com
Source: slideplayer.com
A person drinks 150 x 103 g of water H2O per day. So one molecule of water will have a smaller mass than one molecule of sugar since it contains fewer atoms. By definition one mole would be the same as the atomic mass. You can also use our weight conversion calculators to convert from grams and kilograms to pounds and ounces. Weight of water 2 10079 g 159994 g.
 Source: pinterest.com
Source: pinterest.com
Why is water cohesive. Note that rounding errors may occur so always check the results. Molecular formula of water is H2O. You have one mole of water H 2 moles of atom 2g O 1 mole of atom 16g So adding the two we get the weight of one mole of water which is 18g. If we have one mole of water then we know that it will have a mass of 2 grams for 2 moles of H atoms 16 grams for one mole O atom 18 grams.
 Source: pinterest.com
Source: pinterest.com
If we have one mole of water then we know that it will have a mass of 2 grams for 2 moles of H atoms 16 grams for one mole O atom 18 grams. The SI base unit for amount of substance is the mole. H2O 12 16 18 amu. The periodic table from your book and a calculator will be needed. What is 1 mole of water equal to.
 Source: youtube.com
Source: youtube.com
The SI base unit for amount of substance is the mole. Therefore one mole of water weighs 180152 grams. We assume you are converting between moles Water and gram. So one molecule of water will have a smaller mass than one molecule of sugar since it contains fewer atoms. One mole of water is about 18 milliliters.
 Source: slideplayer.com
Source: slideplayer.com
18 gm per mol is the mass of one mole of water. Therefore one mole of water weighs 180152 grams. Likewise what does 1 mole of water weigh. 1 000 000 000 000. Gram mole is same as mole.
 Source: slideplayer.com
Source: slideplayer.com
Density 1 kgL. 300 x 10-23 c. 1 gram is equal to 0035274 ounces so to get a result in ounces simply multiply the grams by 0035274. Weight of water 2 10079 g 159994 g. 18 amu is the mass of one molecule of water.
 Source: slideplayer.com
Source: slideplayer.com
If I wanted to know how much a dozen eggs weighed I could take the average weight for one egg and multiply by 12. 1 ml x 1gml 1g. So one molecule of water will have a smaller mass than one molecule of sugar since it contains fewer atoms. How much does 1 mole of magnesium weigh in grams. 1 oz 12 12 oz 1 dozen eggs.
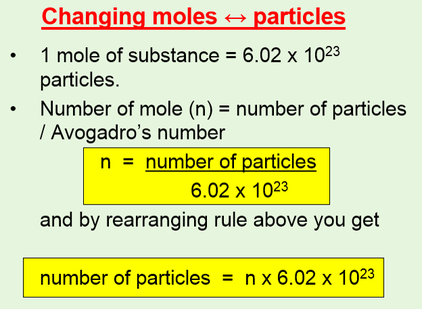 Source: chemistryvce.weebly.com
Source: chemistryvce.weebly.com
Molar mass of H2O 1801528 gmol. Water molecular weight. You have one mole of water H 2 moles of atom 2g O 1 mole of atom 16g So adding the two we get the weight of one mole of water which is 18g. 0000000000000000000000664 g 602200000000000000000000. If I wanted to know how much a dozen eggs weighed I could take the average weight for one egg and multiply by 12.
This site is an open community for users to do sharing their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site convienient, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title how much does 1 mole of water weigh by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






