Your How much does 1 mole of oxygen weigh images are available. How much does 1 mole of oxygen weigh are a topic that is being searched for and liked by netizens now. You can Find and Download the How much does 1 mole of oxygen weigh files here. Get all free photos and vectors.
If you’re searching for how much does 1 mole of oxygen weigh images information related to the how much does 1 mole of oxygen weigh interest, you have visit the ideal site. Our site frequently provides you with hints for seeing the highest quality video and image content, please kindly surf and locate more enlightening video content and graphics that fit your interests.
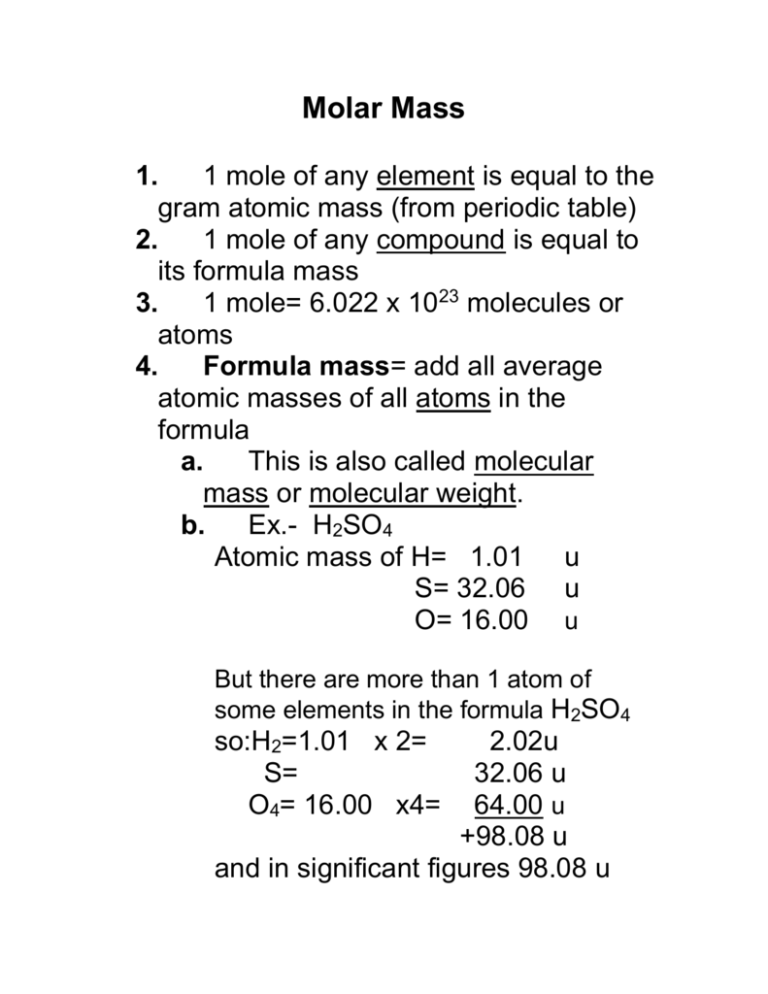
How Much Does 1 Mole Of Oxygen Weigh. Meanwhile a complex molecule like glucose. So the answer is 639976 g. 12 g The mole was defined by International Bureau of Weights and Measures as the amount of substance of a system which contains as. One mole of water weighs 180152 grams.
 Mole And Stoikiometri We Measure Mass In Grams G Ppt Download From slideplayer.com
Mole And Stoikiometri We Measure Mass In Grams G Ppt Download From slideplayer.com
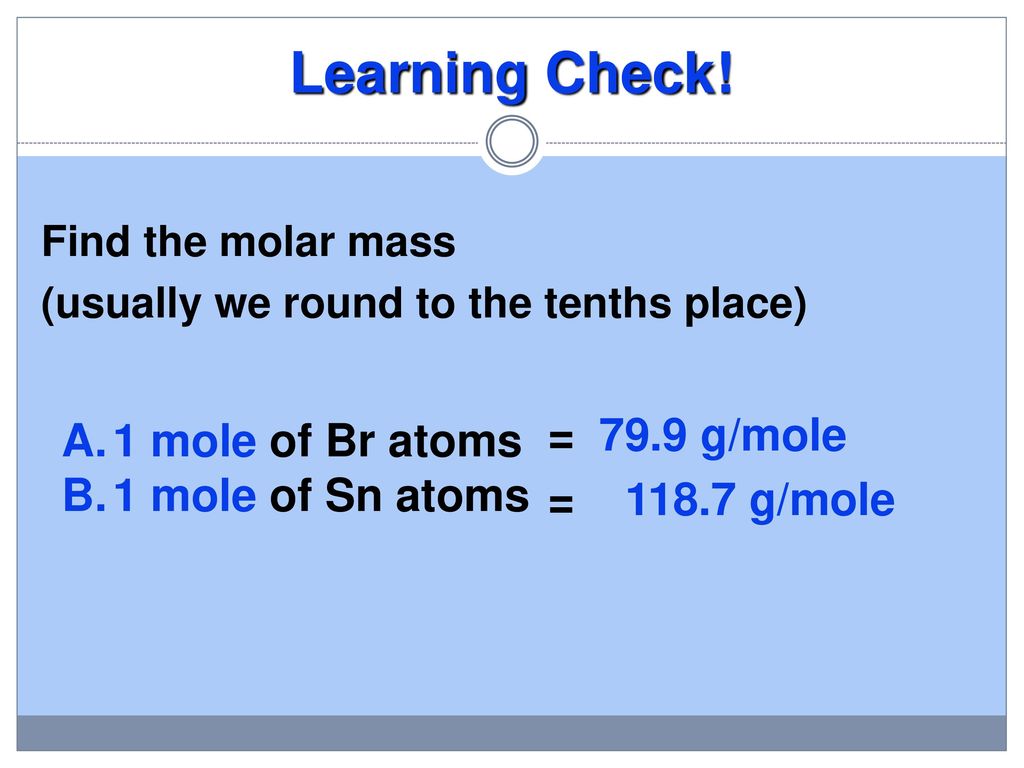
How a lot does 1 mole of carbon dioxide weigh. The SI base unit for amount of substance is the mole. 100000 Calculate the molecular weight of a chemical compound. 12 g The mole was defined by International Bureau of Weights and Measures as the amount of substance of a system which contains as. From here the answer is simple. This means 1 mole of hydrogen weighs 10079 grams and 1 mole of oxygen weighs 159994 grams.
What is the mass of 05 mole of.
Ergo 100 mol of O has a mass of 159949 g usually rounded to 16 g. Just multiply 2 by 319988 g because there are two moles of oxygen. Liquid measured at 1. How much does 1 mole iron weigh. How much does 2 mole of oxygen weigh. How many grams are in a mole of hydrogen.
 Source: studylib.net
Source: studylib.net
Atomic Mass of Atoms. Oxygen molecular weight. What is the mass of 42 mole of oxygen atom. What is the volume of 1 mole of CO2. That said to find the mass of one ATOM we need to convert from moles to atoms as follows.
 Source: slideplayer.com
Source: slideplayer.com
Mass of 0. Density of oxygen is equal to 1429 kgm³. Browse the list of common chemical. Ergo 100 mol of O has a mass of 159949 g usually rounded to 16 g. Just multiply 2 by 319988 g because there are two moles of oxygen.
 Source: slideplayer.com
Source: slideplayer.com
How many moles are 36 gram of water. Oxygen weighs 0001429 gram per cubic centimeter or 1429 kilogram per cubic meter ie. Convert grams Oxygen to moles or moles Oxygen to grams Percent composition by element. Molecular weight of Oxygen or grams The molecular formula for Oxygen is O. One mole of water weighs 180152 grams.
 Source: toppr.com
Source: toppr.com
What is mass of 1 mole of oxygen atom. 100000 Calculate the molecular weight of a chemical compound. Density of oxygen is equal to 1429 kgm³. Oxygen weighs 0001429 gram per cubic centimeter or 1429 kilogram per cubic meter ie. Moles Question Answer How many grams are there in 34 x 1024 molecules of NH3.
 Source: slideplayer.com
Source: slideplayer.com
Convert grams Oxygen to moles or moles Oxygen to grams Percent composition by element. The atomic mass of an element measured in amu is the same as the mass in grams of one mole of an element. 132089 gmol How much does 42 moles of Ca NO3 2 weigh. How much does 1 mole of nitrogen weigh. Weight of water 180152 g.
 Source: youtube.com
Source: youtube.com
How much does 2 mole of oxygen weigh. Note that rounding errors may occur so always check the results. However when you say oxygen do you mean the element O or do you mean the elementary substance O2. At 0C 32F or 27315K at standard atmospheric pressure. A mole is the atomic weight of a molecule of the chemical in grams.
 Source: slideplayer.com
Source: slideplayer.com
What is the mass of 100 mol of oxygen. Convert grams Oxygen to moles or moles Oxygen to grams Percent composition by element. These are the outcomes and the molecular image for every. 639976 g per mole of oxygen. From the periodic table we see the atomic weight of hydrogen is 10079 and the atomic weight of oxygen is 159994.
 Source: slideplayer.com
Source: slideplayer.com
Carbon Dioxide molecular weight. Density of oxygen is equal to 1429 kgm³. A mole is the atomic weight of a molecule of the chemical in grams. Molecular weight of Oxygen or grams The molecular components for Oxygen is O. 16 g mol.
 Source: chemistrygod.com
Source: chemistrygod.com
One mole of water weighs 180152 grams. That is it weighs 16 g. That said to find the mass of one ATOM we need to convert from moles to atoms as follows. What is the mass of 100 mol of oxygen. This means that 1 MOLE of hydrogen atoms will weigh 1008 grams.
 Source: slideplayer.com
Source: slideplayer.com
From the periodic table we see the atomic weight of hydrogen is 10079 and the atomic weight of oxygen is 159994. What is the volume of 1 mole of CO2. What is mass of 1 mole of oxygen atom. In Imperial or US customary measurement system the density is equal to 008921 pound per cubic foot lbft³ or 000082601 ounce per cubic inch ozinch³. Density of oxygen is equal to 1429 kgm³.
 Source: slideplayer.com
Source: slideplayer.com
4401 gmol Carbon dioxideMolar mass. One mole of any element has the same mass in grams as indicated on most periodic tables. What is mass of 1 mole of oxygen atom. Meanwhile a complex molecule like glucose. How much does one mole of atmospheric oxygen weigh O 2 2 x 15999 31998 g from CH 101 at North Carolina State University.
 Source: slideplayer.com
Source: slideplayer.com
Molar mass of O 159994 gmol. This implies 1 mole of hydrogen weighs 10079 grams and 1 mole of oxygen weighs 159994 grams. Grams What is the molar mass of CA NO2 2. Density of oxygen is equal to 1429 kgm³. The atomic mass of an element measured in amu is the same as the mass in grams of one mole of an element.
 Source: pinterest.com
Source: pinterest.com
Oxygen molecular weight. That is it weighs 16 g. One mole of atoms of oxygen has a mass of 16 g as 16 is the atomic weight of oxygen and contains 602 X 1023 atoms of oxygen. From the periodic table we see the atomic weight of hydrogen is 10079 and the atomic weight of oxygen is 159994. Weight of 1 mole of Oxygen The entered amount of Oxygen in various units of amount of substance About Oxygen Oxygen weighs 0001429 gram per cubic centimeter or 1429 kilogram per cubic meter ie.
 Source: courses.lumenlearning.com
Source: courses.lumenlearning.com
This implies 1 mole of hydrogen weighs 10079 grams and 1 mole of oxygen weighs 159994 grams. What is mass of 1 mole of oxygen atom. A gram-mole of oxygen gas which is diatomic weighs about 31998 grams. How much does 1 mole of CO2 weigh. At 0C 32F or 27315K at standard atmospheric pressure.
 Source: studylib.net
Source: studylib.net
One mole of atoms of oxygen has a mass of 16 g as 16 is the atomic weight of oxygen and contains 602 X 1023 atoms of oxygen. This implies 1 mole of hydrogen weighs 10079 grams and 1 mole of oxygen weighs 159994 grams. In Imperial or US customary measurement system the density is equal to 008921 pound per cubic foot lbft³ or 000082601 ounce per cubic inch ozinch³. Meanwhile a complex molecule like glucose. Use this page to learn how to convert between moles Oxygen and gram.
This site is an open community for users to do sharing their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site good, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title how much does 1 mole of oxygen weigh by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






