Your How much does 1 mole of h20 weigh images are ready. How much does 1 mole of h20 weigh are a topic that is being searched for and liked by netizens now. You can Find and Download the How much does 1 mole of h20 weigh files here. Get all free photos and vectors.
If you’re looking for how much does 1 mole of h20 weigh images information related to the how much does 1 mole of h20 weigh topic, you have visit the ideal blog. Our website always provides you with suggestions for viewing the maximum quality video and image content, please kindly surf and find more enlightening video content and images that match your interests.
How Much Does 1 Mole Of H20 Weigh. 1 grams H20 is equal to 004960612734885 mole. Chemistry 19012020 1728 joviecar. This means 1 mole of. You can view more details on each measurement unit.
 Atomic Mass The Atomic Mass Is The Average Mass Of An Atom Ppt Video Online Download From slideplayer.com
Atomic Mass The Atomic Mass Is The Average Mass Of An Atom Ppt Video Online Download From slideplayer.com
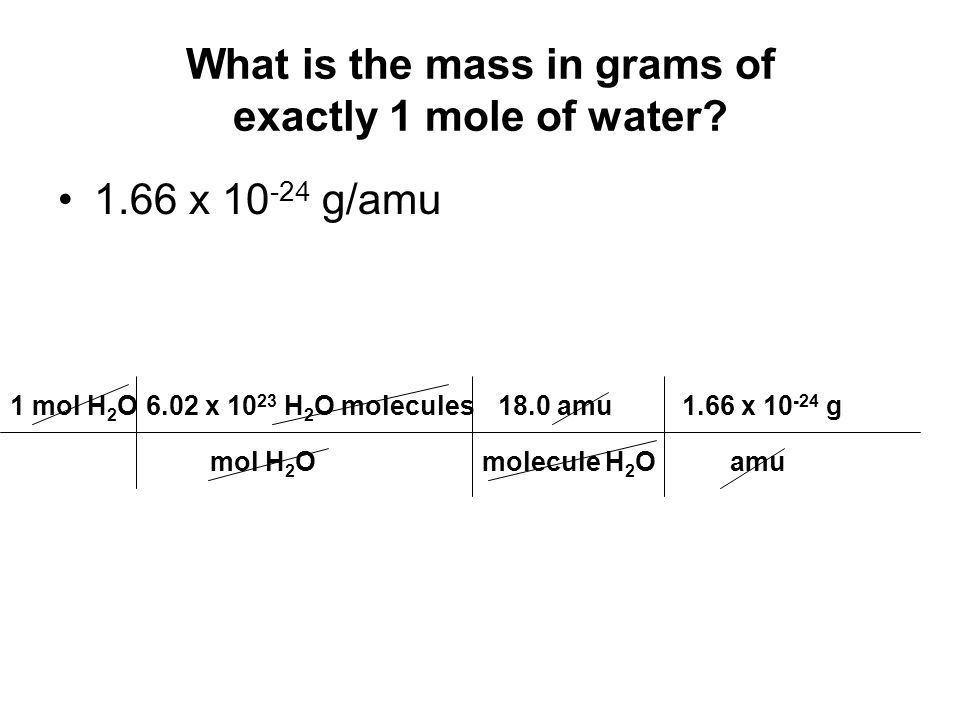
How much does one mole of H2O weigh. H 2 O molecule weight 1801528602214076 10 23 H 2 O molecule weight 29915 10-23 g. 1 grams H2O is the same as 0055508435061792 mole. From the periodic table we see the atomic weight of hydrogen is 10079 and the atomic weight of oxygen is 159994. The periodic table from your book and a calculator will be needed. 2 moles of H2O gets you 36 grams.
PLEASE HELP ASAP.
You might be interested in. How many grams does 400 moles of H20 weigh. Mnmr 4216 72 g. A mole of water molecules would be 2 moles of hydrogen atoms plus 1 mole of oxygen atoms. The SI base unit for amount of substance is the mole. Chemistry 19012020 1728 joviecar.
 Source: toppr.com
Source: toppr.com
Molar mass of H20 kg Molar mass of H20 kg is 201588 gmol. Click to Get Answer. For our practice problem well. 1 mole of oxygen atoms got 16 grams. How many grams does 400 moles of H20 weigh.
 Source: slideplayer.com
Source: slideplayer.com
Alternatively if your reaction took place at standard temperature and pressure 273 K 1 atm then the molar volume is 224 dm3. We assume you are converting between moles H2O and gram. For our practice problem well. Track your food intake exercise sleep and meditation for free. 1 Montrez les réponses.
 Source: clutchprep.com
Source: clutchprep.com
QUESTION 15 How much does 1 mole of water H20 weigh. How much does one mole of H2O weigh. In this video well learn to convert moles of H2O to grams. 1 mole of hydrogen atoms got 1 gram so 2 moles of them got 2 grams. Correct to the nearest integer.
 Source: slideplayer.com
Source: slideplayer.com
In this video well learn to convert moles of H2O to grams. Convert between H20 kg weight and moles. You might be interested in. The atomic mass of each element is normally written the upper right corner of the elements panel. 2 x 1008 15999 18015.
 Source: slideserve.com
Source: slideserve.com
Using the chemical formula of the compound and the periodic table of elements we can add up the atomic weights and calculate molecular weight of the substance. How much in grams does 100 mole of h2o water weigh. 1 Montrez les réponses. Track your food intake exercise sleep and meditation for free. So one molecule of H 2 O weighs 29915 10-23 grams.
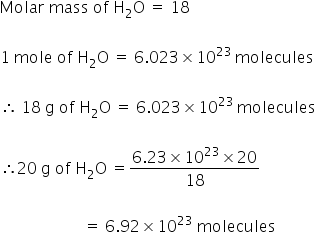 Source: topperlearning.com
Source: topperlearning.com
So one molecule of H 2 O weighs 29915 10-23 grams. Finding molar mass starts with units of grams per mole gmol. QUESTION 15 How much does 1 mole of water H20 weigh. What you are given 03181. PLEASE HELP ASAP.
 Source: slideplayer.com
Source: slideplayer.com
Determined from the sum of potential and kinetic energy in a substance. A mole of water molecules would be 2 moles of hydrogen atoms plus 1 mole of oxygen atoms. Determined from the sum of potential and kinetic energy in a substance. When calculating molecular weight of a chemical compound it tells us how many grams are in one mole of that substance. Get control of 2021.
 Source: youtube.com
Source: youtube.com
1 Montrez les réponses. The volume of one mole of CO2 produced is 24 dm3 at room temperature and pressure. A mole of water molecules would be 2 moles of hydrogen atoms plus 1 mole of oxygen atoms. How much does 1 mole of CO2 weigh. 75 285 Review How much does one mole of H2O weigh.
 Source: pinterest.com
Source: pinterest.com
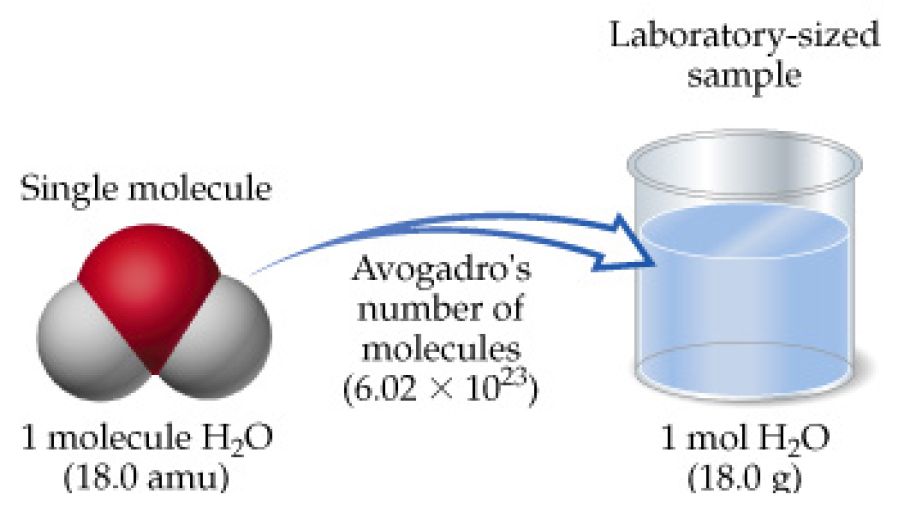
The atomic mass of each element is normally written the upper right corner of the elements panel. 1 mole is equal to 1 moles H20 or 201588 grams. 100 grams H2O 1 mole H2O18016 grams6022 X 10231 mole H2O 334 X 1024 molecules of water Of course you see how many oxygen atoms in that much water. Mnmr 4216 72 g. QUESTION 15 How much does 1 mole of water H20 weigh.
 Source: slideplayer.com
Source: slideplayer.com
Note that rounding errors may occur so always check the results. 100 mol x 1802 g H2O 1 mol H2O 1802 g H20. You can view more details on each measurement unit. Using the chemical formula of the compound and the periodic table of elements we can add up the atomic weights and calculate molecular weight of the substance. QUESTION 15 How much does 1 mole of water H20 weigh.
 Source: socratic.org
Source: socratic.org
1 grams H20 is equal to 004960612734885 mole. 1 grams H2O is the same as 0055508435061792 mole. Note that rounding errors may occur so always check the results. Alternatively if your reaction took place at standard temperature and pressure 273 K 1 atm then the molar volume is 224 dm3. Molecular weight of H20 or mol The SI base unit for amount of substance is the mole.
 Source: slideplayer.com
Source: slideplayer.com
Molecular weight of H20 or grams. Correct to the nearest integer. 1 question What is the molecular weight of one mole of H20. So one molecule of H 2 O weighs 29915 10-23 grams. 1 grams H20 is equal to 004960612734885 mole.
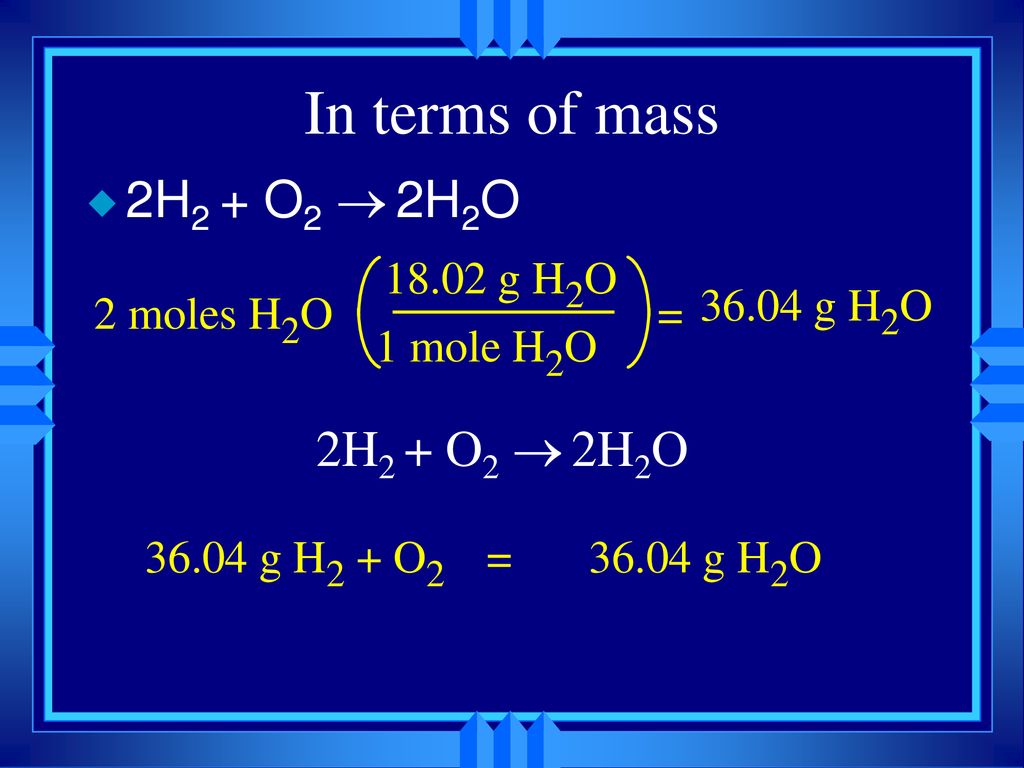 Source: slideplayer.com
Source: slideplayer.com
Using the chemical formula of the compound and the periodic table of elements we can add up the atomic weights and calculate molecular weight of the substance. The volume of one mole of CO2 produced is 24 dm3 at room temperature and pressure. You can view more details on each measurement unit. We assume you are converting between moles H2O and gram. Correct to the nearest integer.

1 mole of hydrogen atoms got 1 gram so 2 moles of them got 2 grams. Track your food intake exercise sleep and meditation for free. When calculating molecular weight of a chemical compound it tells us how many grams are in one mole of that substance. 2 x 1008 15999 18015. Using this constant and the molar mass above the formula to find the weight of one H 2 O molecule is.

QUESTION 15 How much does 1 mole of water H20 weigh. 2 moles of H2O gets you 36 grams. When calculating molecular weight of a chemical compound it tells us how many grams are in one mole of that substance. 1 mole of oxygen atoms got 16 grams. Chemistry 19012020 1728 joviecar.
This site is an open community for users to share their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site beneficial, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title how much does 1 mole of h20 weigh by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






