Your How much does 1 mole of carbon weigh images are ready in this website. How much does 1 mole of carbon weigh are a topic that is being searched for and liked by netizens today. You can Download the How much does 1 mole of carbon weigh files here. Download all royalty-free photos and vectors.
If you’re looking for how much does 1 mole of carbon weigh images information related to the how much does 1 mole of carbon weigh keyword, you have visit the right site. Our website frequently provides you with suggestions for downloading the maximum quality video and image content, please kindly surf and find more informative video articles and graphics that match your interests.
How Much Does 1 Mole Of Carbon Weigh. When scientists do experiments with substances elements. Avogadros number 6023 X1023. Molecular mass of C4H10. A mole of carbon contains 60221023 atoms.

Use this page to learn how to convert between moles Carbon and gram. We know that one mole of gas takes up 24 litres at room temperature and pressure. When scientists do experiments with substances elements. 2 mol H 1 mol O How much does H 2O weigh. Carbon dioxide weighs 0001836 gram per cubic. Formula weights the sum of the atomic weights of the elements in the formula each taken the number of times the.
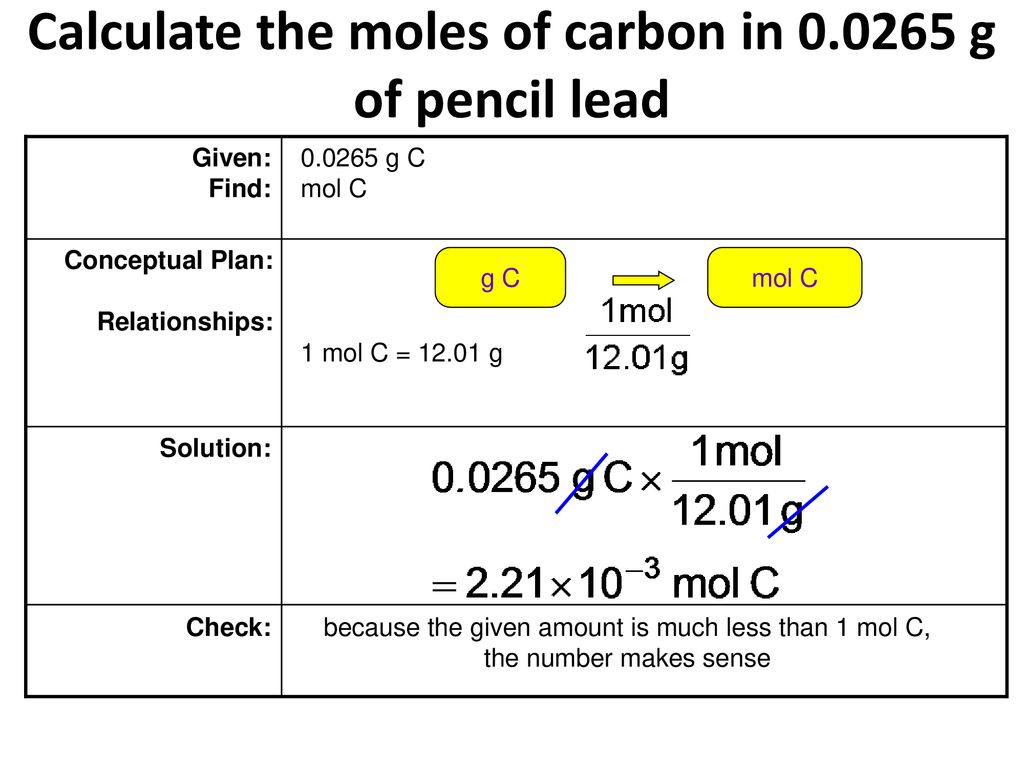
One carbon atom has a mass of 1200 amu see the Periodic Table.
A mole of carbon contains 60221023 atoms. 100mol of carbon-12 atoms has a mass of 120g and contains 60221023 atoms. Carbon dioxide weighs 0001836 gram per cubic. How much does one mole of carbon weigh. Molar mass of C 120107 gmol. Molecular mass of C4H10.
 Source: slideplayer.com
Source: slideplayer.com
So a single carbon atom weighs 12 amu while a mole of carbon atoms weighs 1201gmol. Mass of 1 atom of carbon 602210 2312. How much does one mole of hexane weigh. I told you that one mole of carbon dioxide weighs 44 grams so if you divide 44 million grams by 44 that means you must have ten to the power of five moles of carbon dioxide that youve made through the year. 1 mole is equal to 1 moles SIO2 or 19096827 grams.

TAP THE ARROWS BELOW TO ADVANCE. So instead we talk about a mole of molecules which means 6022 10 23 or about 60 trillion trillion. Note that rounding errors may occur so always check the results. You just studied 22 terms. A mole of carbon contains 60221023 atoms.
 Source: youtube.com
Source: youtube.com
The molar mass is the mass of one mole of any particular substance. By its definition one mole of an element has the same weight in grams as one atom of the element weighs in amu. The unit suggests that every mole of carbon weighs 1201 grams. Hence the correct option is A. Therefore one mole of carbon atoms has a mass of 1200 grams.
 Source: slideplayer.com
Source: slideplayer.com
Use this page to learn how to convert between moles Carbon and gram. A mole of apples has 6022 x 10 23 apples. Hence the correct option is A. Carbon molecular weight. By its definition one mole of an element has the same weight in grams as one atom of the element weighs in amu.
 Source: youtube.com
Source: youtube.com
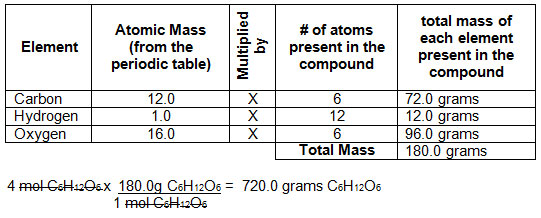
How many atoms are in 12 moles carbon. This number is 6022 x 10 23 carbon atoms. I told you that one mole of carbon dioxide weighs 44 grams so if you divide 44 million grams by 44 that means you must have ten to the power of five moles of carbon dioxide that youve made through the year. 4 x 1200g 10 x101g 1 mole of C4H10 581 g. How many atoms are in 12 moles carbon.
 Source: chem.fsu.edu
Source: chem.fsu.edu
A single mole is set to the number of particles found in 12000 grams of carbon-12. In SI units a mole is defined by the amount of carbon atoms there are in 12g of carbon-12 the nucleus of a carbon-12 atom contains 6 protons and 6 neutrons hence carbon-12. Number of atoms in C4H10. When scientists do experiments with substances elements. Hence the correct option is A.

Carbon weighs 2266 gram per cubic centimeter or 2 266 kilogram. Carbon dioxide weighs 0001836 gram per cubic. Carbon molecular weight. Mass of one mole of carbon atom 12 g. How much does 1 mole of carbon-12 weigh grams.
 Source: youtube.com
Source: youtube.com
Mass of one mole of carbon atom 12 g. This is one of the reasons why a diamond being composed almost entirely of carbon atoms was chosen. You just studied 22 terms. In SI units a mole is defined by the amount of carbon atoms there are in 12g of carbon-12 the nucleus of a carbon-12 atom contains 6 protons and 6 neutrons hence carbon-12. But in the lab scientists use grams its more practical.
 Source: dynamicscience.com.au
Source: dynamicscience.com.au
Mass of one mole of carbon atom 12 g. Avogadros number 6023 X1023. One moles of the substance weighs approximately 1201 gmole. Why are moles removed. Number of atoms in C4H10.
 Source: chemistry.wustl.edu
Source: chemistry.wustl.edu
TAP THE ARROWS BELOW TO ADVANCE. How many atoms are in 12 moles carbon. Cosmetic reasons reduce irritation and discomfort from rubbing against clothing suspected of. You just studied 22 terms. In CARBON material it has also 60221023 atoms in one mole of it that is equivalent to 12 grams as it has atomic weight 12amu.

The number of atoms present in carbon specifically Carbon-12 is the number of atoms present in 1 gram of the substance. The molecular formula for Carbon is C. 100mol of carbon-12 atoms has a mass of 120g and contains 60221023 atoms. The molar mass is the mass of one mole of any particular substance. Carbon weighs 2266 gram per cubic centimeter or 2 266 kilogram.
 Source: chemistry.wustl.edu
Source: chemistry.wustl.edu
The SI base unit for amount of substance is the mole. Note that rounding errors may occur so always check the results. The number 6022 x 10 23 is known as Avogadros Number. What is the weight of 2 moles of CO2. How many atoms are in a gram of a compound.
 Source: brainly.in
Source: brainly.in
A single mole is set to the number of particles found in 12000 grams of carbon-12. But in the lab scientists use grams its more practical. 100 mole of any element has a mass numerically equal to its atomic mass in grams and contains 60221023 particles. The molecular formula for Carbon is C. If one mole of carbon atoms weighs 12 grams what is the mass in grams of 1 atom of carbon.
 Source: chem.libretexts.org
Source: chem.libretexts.org
Formula weights the sum of the atomic weights of the elements in the formula each taken the number of times the. The number of atoms present in carbon specifically Carbon-12 is the number of atoms present in 1 gram of the substance. 100mol of carbon-12 atoms has a mass of 120g and contains 60221023 atoms. The number 6022 x 10 23 is known as Avogadros Number. Kind of like a dozen eggs but a much bigger number.
 Source: cdli.ca
Source: cdli.ca
How much does one sucrose molecule weigh in grams. 4 x 1200g 10 x101g 1 mole of C4H10 581 g. The molar mass is the mass of one mole of any particular substance. How many atoms are in 12 moles carbon. Molecular mass of C4H10.
This site is an open community for users to do sharing their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site value, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title how much does 1 mole of carbon weigh by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






