Your How many particles in one mole of nacl images are ready in this website. How many particles in one mole of nacl are a topic that is being searched for and liked by netizens now. You can Download the How many particles in one mole of nacl files here. Find and Download all free photos.
If you’re searching for how many particles in one mole of nacl images information related to the how many particles in one mole of nacl topic, you have visit the ideal site. Our site always gives you suggestions for refferencing the maximum quality video and image content, please kindly search and find more enlightening video content and graphics that match your interests.
How Many Particles In One Mole Of Nacl. Molecular weight of NaCl or mol This compound is also known as Sodium Chloride. How many moles are in 2 grams of NaOH. Like if someone asks us to determine the number of moles in say 7233 grams of NaCl. How many moles are in the substance.
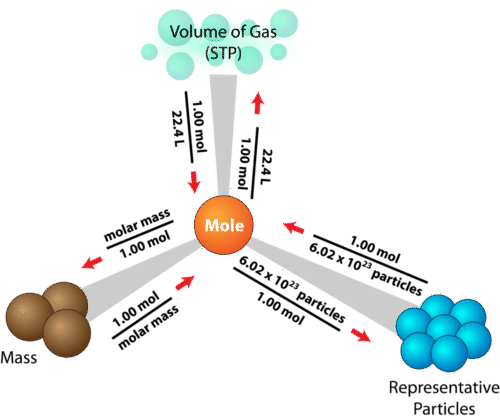
 Mole Definition Molar Mass Avogadro S Constant Chemistrygod From chemistrygod.com
Mole Definition Molar Mass Avogadro S Constant Chemistrygod From chemistrygod.com
Mol of NaCl where a mole is 60221023 molecules. 1 mole of water H2O. How many moles are in the substance. Which example has 121024 oxygen atoms. You can view more details on each measurement unit. 0 2 3 1 0 2 3 molecules.
1 mole of peroxide H2O2.
Mol of NaCl where a mole is 60221023 molecules. The SI base unit for amount of substance is the mole. Thus 585 g of NaCl corresponds to 1 mole of NaCl and contains 6023 1 0 23 displaystyle 6023 imes 1023 6. All depends on what you call particles. How many moles are in the substance. 5 5 8.
 Source: slideplayer.com
Source: slideplayer.com
1 mole of water H2O. There is 171 moles in 100 grams of NaCl. One mole of Na and one mole of Cl-. The answer is 5844277. For an experiment you need to dissolve 010 mole of NaCl in 1 liter of water.
 Source: chemiris.labs.brocku.ca
Source: chemiris.labs.brocku.ca
MOLARITY is moles per liter molL we represent it wcapital M eg. Which example has 121024 oxygen atoms. An osmole Osmol is 1 mol of particles that contribute to the osmotic pressure of a solution. Use this page to learn how to convert between moles NaCl and gram. You find this by taking the molar mass of Na which is 2299 and Cl which is 3545 and you add them together then divide by 100.
 Source: youtube.com
Source: youtube.com
The answer is 5844277. A 1 M solution of glycerol indicates that there is 1 mole of solute per liter of solution. How much NaCl must you weigh out. And if 1 mole has 602 x 1023 formula units the that little crystal would have 103 x 1019 formula units. Note that rounding errors may occur so always check the results.
 Source: slideplayer.com
Source: slideplayer.com
5 5 8. A solution of 1 molL NaCl has an osmolarity of 2 OsmolL. A 1M solution has 1 molL so 1 L has 1 mol 2L has 2 mol 05L has 05 mol etc The mole fraction aka molar fraction is the relative amount of 1. Moles 50585 0855 moles 3sf. MOLARITY is moles per liter molL we represent it wcapital M eg.
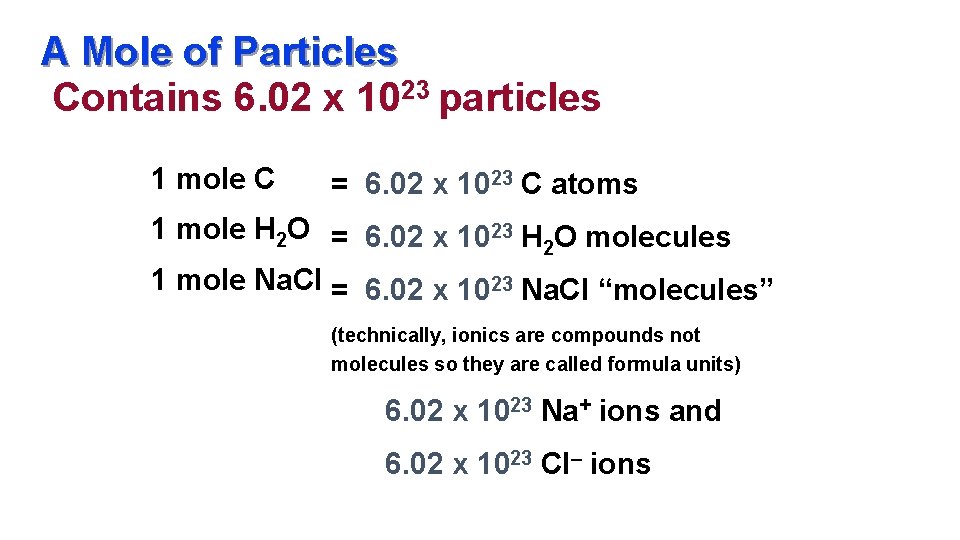 Source: slidetodoc.com
Source: slidetodoc.com
1 mole is equal to 1 moles NaCl or 5844277 grams. 0 2 3 1 0 2 3 molecules. Molecular weight of NaCl or mol This compound is also known as Sodium Chloride. You find this by taking the molar mass of Na which is 2299 and Cl which is 3545 and you add them together then divide by 100. Note that rounding errors may occur so always check the results.
 Source: slideplayer.com
Source: slideplayer.com
All depends on what you call particles. Na 05Cl₂ NaCl. How many moles are in 2 grams of NaOH. A substance has half the number of particles as 12 grams of carbon-12. Therefore the type of particles needs to be specified carefully.
 Source: chemistrygod.com
Source: chemistrygod.com
The SI base unit for amount of substance is the mole. How many moles are in a gram. Therefore Total 5 moles of ions will be there. For an experiment you need to dissolve 005 mole of NaCl in one liter of water. Thus 585 g of NaCl corresponds to 1 mole of NaCl and contains 6023 1 0 23 displaystyle 6023 imes 1023 6.
 Source: slidetodoc.com
Source: slidetodoc.com
A substance has twice the number of particles as 12 grams of carbon-12. We assume you are converting between grams NaCl and mole. One mole is equal to 6022141791023 atoms or other elementary units such as molecules. If it is in aqueous solution all diluted you have 2732 moles of ions. 2 moles of anything contain 2 6022 10²³ units atoms molecules etc.
 Source: slideplayer.com
Source: slideplayer.com
Mol of NaCl where a mole is 60221023 molecules. Its numerical value is 602225 1023. 0 2 3 1 0 2 3 molecules. Use this page to learn how to convert between moles NaCl and gram. Therefore Total 5 moles of ions will be there.
 Source: studylib.net
Source: studylib.net
Type in your own numbers in the form to convert the units. Therefore Total 5 moles of ions will be there. You can view more details on each measurement unit. Na has an atomic mass of 23 and Cl is 355. Thus 585 g of NaCl corresponds to 1 mole of NaCl and contains 6.
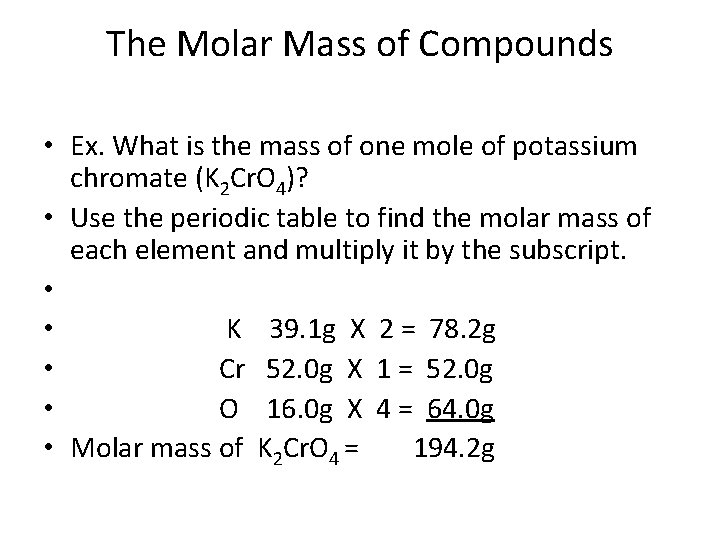 Source: slidetodoc.com
Source: slidetodoc.com
Thus each mole of NaCl becomes two osmoles in solution. One mole is equal to 6022141791023 atoms or other elementary units such as molecules. B 1 mole of molecular substances contains 602 10 23 molecules. One mole amount of any substance that contains as many particles of that substance as there are atoms of C in 12 g pure Carbon-12. An osmole Osmol is 1 mol of particles that contribute to the osmotic pressure of a solution.
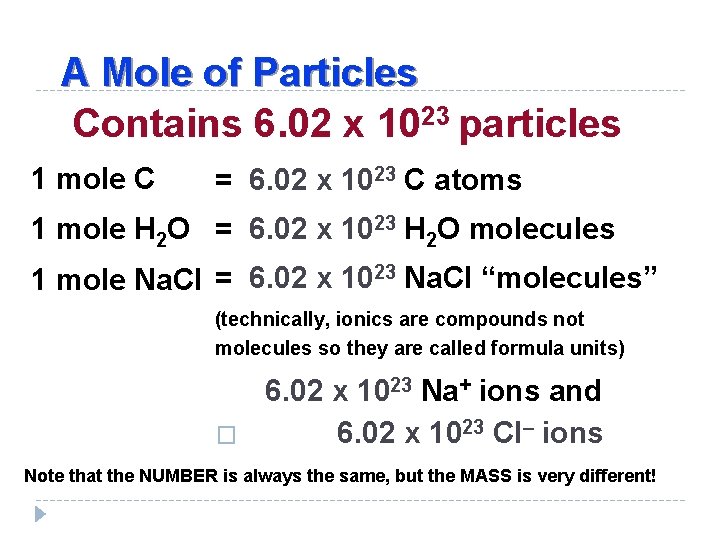 Source: slidetodoc.com
Source: slidetodoc.com
If it is in aqueous solution all diluted you have 2732 moles of ions. You find this by taking the molar mass of Na which is 2299 and Cl which is 3545 and you add them together then divide by 100. Thus each mole of NaCl becomes two osmoles in solution. Therefore Total 5 moles of ions will be there. Now In 25mol of NaCl there is 25 moles of Na and 25moles of Cl- ions.
 Source: slidetodoc.com
Source: slidetodoc.com
A 1 mole of atomic substances contains 602 10 23 atoms. To find moles divide atoms by Avogadros number. The molar mass of NaCl is 2 3 3 5. If I take NaCl as a molecule then there are 2 Na atoms and 2 Cl atoms in both sides or one Na atom and 05 Cl atom in both sides. How many grams NaCl in 1 mol.
 Source: slidetodoc.com
Source: slidetodoc.com
There is 171 moles in 100 grams of NaCl. 2 moles of anything contain 2 6022 10²³ units atoms molecules etc. We assume you are converting between grams NaCl and mole. If I take NaCl a mole of molecules then there are 2 moles of Na and 2 moles of Cl₂ molecules in both sides or 1 mole of Na and 0s moles of Cl₂ molecules present. 5 5 8.
 Source: slideplayer.com
Source: slideplayer.com
For an experiment you need to dissolve 010 mole of NaCl in 1 liter of water. Putting all of this into our moles equation gives us the answer. 1 grams NaCl is equal to 0017110756386119 mole. MOLARITY is moles per liter molL we represent it wcapital M eg. For example 1 mole of zinc contains 602 10 23 zinc atoms.
This site is an open community for users to do submittion their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site convienient, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title how many particles in one mole of nacl by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.