Your How many particles are there in one mole of carbon images are available. How many particles are there in one mole of carbon are a topic that is being searched for and liked by netizens now. You can Download the How many particles are there in one mole of carbon files here. Get all free images.
If you’re searching for how many particles are there in one mole of carbon pictures information related to the how many particles are there in one mole of carbon keyword, you have come to the ideal blog. Our website always provides you with suggestions for downloading the maximum quality video and image content, please kindly search and locate more informative video articles and images that match your interests.
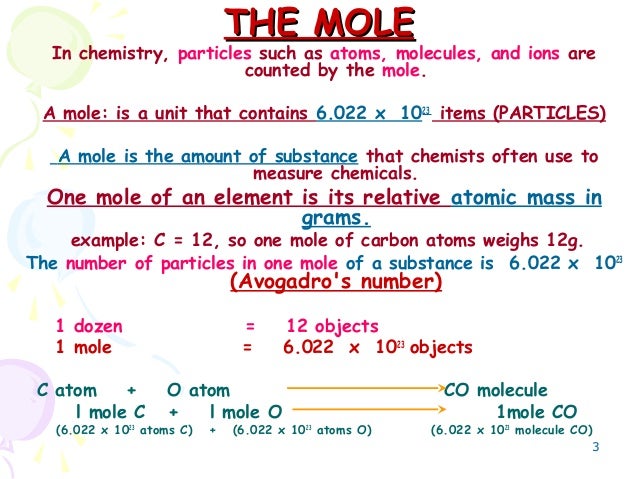
How Many Particles Are There In One Mole Of Carbon. In both cases that is the mass of 602 10 23 representative particles. 1 mole 6022 x 1023 of the representative particles. Number of entities as there are atoms in 12 g of carbon-12. The Avogadro number One mole of atoms contains 6 x.
 How Many Carbon Atoms Are Present In A Mol Clutch Prep From clutchprep.com
How Many Carbon Atoms Are Present In A Mol Clutch Prep From clutchprep.com
Foods Nutrients and Calories. 1 mole of particles 602 x 1023 particles. 6022 x 1023 is called Avogadros number. Treat it like a very large dozen. A sample of naturally occurring carbon has a mass of 1732 grams. A mole of helium contains 602 x 1023 atoms of helium.
The mass in grams of one mole of that compound.
Calculate the number of moles of carbon in this sample. 6022 x 1023 atoms of an element Avogadros number. 6022 10 23 atoms 1 mol or 1 mol. It is the number of representative particles carbon atoms in exactly 12 grams of pure carbon-12. 1 mole contains 6022 x 10 23 entities Avogadros number Concept 1. Or electrons in a substance.

The mass in grams of one mole of that compound. The molar mass is the amount of grams in one mole of a substance. One mole is the Avogadro number of particles atoms molecules ions. 1 mole contains 6022 x 10 23 entities Avogadros number Concept 1. The Avogadro number One mole of atoms contains 6 x.
 Source: youtube.com
Source: youtube.com
Calculate the number of moles of carbon in this sample. Exactly 12 g of carbon-12 contains 6022 x 10 23 atoms. Atomicity of Carbon in C2H6 is 2 as there are 2 carbon atoms in 1 molecule of C2H6. Calculate the number of moles of carbon in this sample. Calculate the mass of one trillion molecules of oxygen gas.
 Source: chemistry.wustl.edu
Source: chemistry.wustl.edu
A sample of naturally occurring carbon has a mass of 1732 grams. People also ask how many particles are in 3 moles. 1 mole 602 x 1023 particles of anything countable. Carbon atoms in exactly 12 grams of carbon-12. A mole of carbon contains 602 x 1023 atoms of carbon.

This number is known as Avogadros number. While a dozen is only 12 particles a mole is a much larger number602 x 1023 particles. People also ask how many particles are in 3 moles. 6022 x 1023 atoms of an element Avogadros number. Hence the number of atoms in 01 m o l of carbon atom is 01 N A atoms or 6022 10 22 atoms.
 Source: clutchprep.com
Source: clutchprep.com
6022 x 1023 is called Avogadros number. One mole is the Avogadro number of particles atoms molecules ions. A mole of carbon contains 602 x 1023 atoms of carbon. The molar masses are 4401 gmol and 7804 gmol respectively. 6022 10 23 atoms 1 mol or 1 mol.
 Source: slideplayer.com
Source: slideplayer.com
16 grams of Oxygen would be one mole of Oxygen atoms and so forth. In general 1 mole 1 molar mass 6022 x 1023 atoms molecules or ions. The molar masses are 4401 gmol and 7804 gmol respectively. The symbol of mole is mol. 6022 10 23 atoms 1 mol or 1 mol.
 Source: toppr.com
Source: toppr.com
Known number of C atoms 472 10 24 1 mole 602 10 23 atoms Unknown One conversion factor will allow us to convert from the number of C atoms to moles of C atoms. Think about your result. The mass in grams of one mole of that compound. The number of atoms of carbon-12 present in this one mole sample is 6022 136 7 x 10 23. One mole of carbon dioxide molecules has a mass of 4401 g while one mole of sodium sulfide formula units has a mass of 7804 g.
 Source: clutchprep.com
Source: clutchprep.com
This number is known as Avogadros number. 1 mole of C2H6 Na 60231023 molecules of C2H6 by the formula No of moles Given particles or atoms or molecules Avogadro constant Atomicity number of atoms. Known number of C atoms 472 10 24 1 mole 602 10 23 atoms Unknown One conversion factor will allow us to convert from the number of C atoms to moles of C atoms. How many molecules of gas were produced. A mole of sodium contains 602 x 1023 atoms of sodium.
 Source: youtube.com
Source: youtube.com
One mole is defined as the amount of substance that contains as many particles as the number of atoms in exactly 12 g of carbon-12 which is 602 10 23 particles. Calculate the number of moles of carbon in this sample. The mass in grams of one mole of that compound. In general 1 mole 1 molar mass 6022 x 1023 atoms molecules or ions. Number of mol is calculated by ratio of given mass to the molar mass.
 Source: chem.libretexts.org
Source: chem.libretexts.org
List the known quantities and plan the problem. One mole of carbon dioxide molecules has a mass of 4401 g while one mole of sodium sulfide formula units has a mass of 7804 g. This number is known as Avogadros number. The symbol of mole is mol. Treat it like a very large dozen.

The amount of moles in a substance can be determined using that substances molar mass. The molar masses are 4401 gmol and 7804 gmol respectively. How many particles are in 5. Carbon atoms in exactly 12 grams of carbon-12. Calculate the number of moles in.
 Source: slideshare.net
Source: slideshare.net
Remember always 1 mole of any substance 6023 1023 particlesatomsionsmolecules So 1 mole of CO2 will have 6023 x1023 molecules Cheers. The amount of material counting 602214 1023 particles The value of the mole is equal to the number of atoms in exactly 12 grams of pure carbon-12. A sample of naturally occurring carbon has a mass of 1732 grams. The amount of moles in a substance can be determined using that substances molar mass. One mole of carbon dioxide molecules has a mass of 4401 g while one mole of sodium sulfide formula units has a mass of 7804 g.
 Source: courses.lumenlearning.com
Source: courses.lumenlearning.com
One mole is the Avogadro number of particles atoms molecules ions. A mole of anything has 60221367 x 1023 particles. A mole of helium contains 602 x 1023 atoms of helium. Calculate the number of moles in. Or electrons in a substance.

Molecule formula unit ion atom etc Hints. Foods Nutrients and Calories. 1 mole of C2H6 Na 60231023 molecules of C2H6 by the formula No of moles Given particles or atoms or molecules Avogadro constant Atomicity number of atoms. A mole of anything has 60221367 x 1023 particles. Remember always 1 mole of any substance 6023 1023 particlesatomsionsmolecules So 1 mole of CO2 will have 6023 x1023 molecules Cheers.
 Source: sites.google.com
Source: sites.google.com
1 mole of C2H6 Na 60231023 molecules of C2H6 by the formula No of moles Given particles or atoms or molecules Avogadro constant Atomicity number of atoms. Elements generally exist as the particles we call atoms. How many molecules of gas were produced. 1 mole of C2H6 Na 60231023 molecules of C2H6 by the formula No of moles Given particles or atoms or molecules Avogadro constant Atomicity number of atoms. One mole is the Avogadro number of particles atoms molecules ions.
This site is an open community for users to share their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site adventageous, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title how many particles are there in one mole of carbon by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.