Your How many moles per liter of water images are available. How many moles per liter of water are a topic that is being searched for and liked by netizens now. You can Download the How many moles per liter of water files here. Find and Download all royalty-free vectors.
If you’re looking for how many moles per liter of water images information related to the how many moles per liter of water keyword, you have come to the right site. Our site always gives you suggestions for refferencing the highest quality video and picture content, please kindly search and locate more informative video articles and images that match your interests.
How Many Moles Per Liter Of Water. To get the number of moles per liter of water we divide the mass of 1L of water 1000g by the mass of a mole of water 18g mole. Molelitre or molmL The SI derived unit for amount-of-substance concentration is the molecubic meter. There are 55346 moles in 1 liter of pure water. 1500 ml 1 gml 18 gmol 833 moles.
 Two Moles Per Liter T Shirt Snorgtees In 2021 Science Humor Funny Science Jokes Science Tshirts From pinterest.com
Two Moles Per Liter T Shirt Snorgtees In 2021 Science Humor Funny Science Jokes Science Tshirts From pinterest.com
We assume you are converting between molelitre and molemilliliter. This is calculated by dividing the density of water which is 99707 grams per liter by the molecular mass of water which is 1802 grams per mole. The calculator below uses the formula to convert liters to moles and to convert moles to liters where is 22413962 Convert moles to liters and liters to moles Convert. 1000 18 55 5 9 moles. The density of water is 1gml so if you do dimensionalanalysis you can find that in 1 L of water there is 5556 molwhich means the molarity of water is 5556. Knowing that water is 18 grams per mole you can do the math.
1000 18 5556 moles per kg Next we multiply by Avogadros number to obtain the final answer.
1 mol of water H20 18 g. The density of water is 1gml so if you do dimensionalanalysis you can find that in 1 L of water there is 5556 molwhich means the molarity of water is 5556. It just so happens that at standard temperature and pressure the density of water is 1g per mL. 1500 ml 1 gml 18 gmol 833 moles. First find the number of moles by dividing a kilogram which is the mass of 1 liter of water by the molar weight. The key to the conversion is realizin.
 Source: pinterest.com
Source: pinterest.com
A liter of water is 1000g of water. Therefore one liter of water contains 1000 grams 1 mole 18 grams 5556 moles of water. A liter of water is 1000g of water. How many mols of HCl are required to make 600mL of a 15M solution. Knowing that water is 18 grams per mole you can do the math.
 Source: redbubble.com
Source: redbubble.com
1000 18 5556 moles per kg Next we multiply by Avogadros number to obtain the final answer. 1000 g x 1 mol18 g of water in 1 liter of water. To get the number of moles per liter of water we divide the mass of 1L of water 1000g by the mass of a mole of water 18g mole. First find the number of moles by dividing a kilogram which is the mass of 1 liter of water by the molar weight. Molelitre or molmL The SI derived unit for amount-of-substance concentration is the molecubic meter.
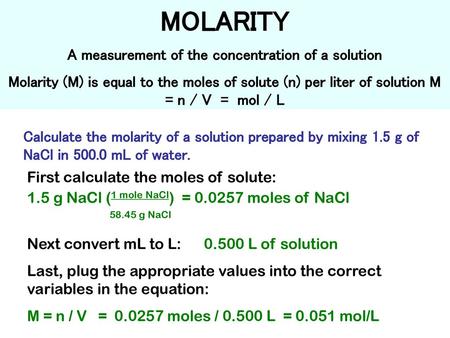 Source: slideplayer.com
Source: slideplayer.com
You can view more details on each measurement unit. Therefore one liter of water contains 1000 grams 1 mole 18 grams 5556 moles of water. To convert grams to moles start by multiplying the number of atoms by the atomic weight for each element in the compound. 1000 18 55 5 9 moles. A liter of water is 1000g of water.
 Source: redbubble.com
Source: redbubble.com
The answer is 1000. The key to the conversion is realizin. 1ml 1g1ml 1mol18g 118 moles of water. 1500 ml 1 gml 18 gmol 833 moles. Because there are 6022 1023 molecules in a mole the number of molecules of water in a liter is 6022 1023 5556 approximately 3345 1025 molecules of water.
 Source: slideplayer.com
Source: slideplayer.com
The calculator below uses the formula to convert liters to moles and to convert moles to liters where is 22413962 Convert moles to liters and liters to moles Convert. The answer is 1000. 1000 18 moles. Knowing that water is 18 grams per mole you can do the math. To get the number of moles per liter of water we divide the mass of 1L of water 1000g by the mass of a mole of water 18g mole.
 Source: pinterest.com
Source: pinterest.com
Because there are 6022 1023 molecules in a mole the number of molecules of water in a liter is 6022 1023 5556 approximately 3345 1025 molecules of water. To get the number of moles per liter of water we divide the mass of 1L of water 1000g by the mass of a mole of water 18g mole. To convert grams to moles start by multiplying the number of atoms by the atomic weight for each element in the compound. First find the number of moles by dividing a kilogram which is the mass of 1 liter of water by the molar weight. This is calculated by dividing the density of water which is 99707 grams per liter by the molecular mass of water which is 1802 grams per mole.
 Source: researchgate.net
Source: researchgate.net
This is calculated by dividing the density of water which is 99707 grams per liter by the molecular mass of water which is 1802 grams per mole. 1g 1mol18g 118 moles about 0056 moles in 1ml 1g of water. This can be done in one step as. Therefore one liter of water contains 1000 grams 1 mole 18 grams 5556 moles of water. 1000 18 moles.
 Source: redbubble.com
Source: redbubble.com
This is calculated by dividing the density of water which is 99707 grams per liter by the molecular mass of water which is 1802 grams per mole. This is calculated by dividing the density of water which is 99707 grams per liter by the molecular mass of water which is 1802 grams per mole. It just so happens that at standard temperature and pressure the density of water is 1g per mL. The answer is 1000. The number of molecules in a particular volume depends on the density of the liquid but lets assume you meant at STP where water is about 1 gml.
 Source: pinterest.com
Source: pinterest.com
The atomic mass of water is 18 grams per mole. 1000 18 moles. To get the number of moles per liter of water we divide the mass of 1L of water 1000g by the mass of a mole of water 18g mole. This can be done in one step as. The atomic mass of water is 18 grams per mole.
 Source: redbubble.com
Source: redbubble.com
How many molelitre in 1 molmL. 1000 18 55 5 9 moles. 1ml 1g1ml 1mol18g 118 moles of water. You can view more details on each measurement unit. Density of water 1gcm³ 1L 1000 cm³ mass of 1L water densityvolume 1gcm³ 1000cm³ 1000g molar mass of water 18g number of moles in 1000g.
 Source: discoveryexpresskids.com
Source: discoveryexpresskids.com
15molliter 06L 09 mols. The atomic mass of water is 18 grams per mole. The density of water is 1gml so if you do dimensionalanalysis you can find that in 1 L of water there is 5556 molwhich means the molarity of water is 5556. Therefore one liter of water contains 1000 grams 1 mole 18 grams 5556 moles of water. How do you convert to moles.
 Source: pinterest.com
Source: pinterest.com
1g 1mol18g 118 moles about 0056 moles in 1ml 1g of water. To get the number of moles per liter of water we divide the mass of 1L of water 1000g by the mass of a mole of water 18g mole. There are 55346 moles in 1 liter of pure water. It just so happens that at standard temperature and pressure the density of water is 1g per mL. Therefore one liter of water contains 1000 grams 1 mole 18 grams 5556 moles of water.
 Source: qwertee.tumblr.com
Source: qwertee.tumblr.com
How many molelitre in 1 molmL. It just so happens that at standard temperature and pressure the density of water is 1g per mL. 1000 g x 1 mol18 g of water in 1 liter of water. Therefore one liter of water contains 1000 grams 1 mole 18 grams 5556 moles of water. To convert grams to moles start by multiplying the number of atoms by the atomic weight for each element in the compound.
 Source: pinterest.com
Source: pinterest.com
1ml 1g1ml 1mol18g 118 moles of water. You can view more details on each measurement unit. The density of water is 1gml so if you do dimensionalanalysis you can find that in 1 L of water there is 5556 molwhich means the molarity of water is 5556. 1000 18 moles. The key to the conversion is realizin.
 Source: pinterest.com
Source: pinterest.com
1000 18 moles. Knowing that water is 18 grams per mole you can do the math. 1000 18 55 5 9 moles. Therefore one liter of water contains 1000 grams 1 mole 18 grams 5556 moles of water. Molelitre or molmL The SI derived unit for amount-of-substance concentration is the molecubic meter.
This site is an open community for users to do sharing their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site value, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title how many moles per liter of water by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






