Your How many moles of oxygen atoms are in 1 mole of cuno32 images are ready. How many moles of oxygen atoms are in 1 mole of cuno32 are a topic that is being searched for and liked by netizens today. You can Get the How many moles of oxygen atoms are in 1 mole of cuno32 files here. Find and Download all royalty-free photos and vectors.
If you’re looking for how many moles of oxygen atoms are in 1 mole of cuno32 images information connected with to the how many moles of oxygen atoms are in 1 mole of cuno32 topic, you have come to the ideal blog. Our website frequently gives you suggestions for viewing the highest quality video and image content, please kindly surf and locate more enlightening video content and images that fit your interests.
How Many Moles Of Oxygen Atoms Are In 1 Mole Of Cuno32. This could be converted. Click to see full answer. 1 See answer Advertisement Advertisement sboula is waiting for your help. How many atoms are present in 16 grams of oxygen.

1 grams CuNO32 is equal to 00053317466055435 mole. So if 1 g Carbon atom of CO combine with 133g of Oxygen. BSc honours from Carleton University. You can view more details on each measurement unit. 0448 moles O atoms x 602x1023 atomsmole 270x1023 atoms of. Molecular weight of CuNo3 or grams The SI base unit for amount of substance is the mole.
CHEBI78036 copperII nitrate An inorganic nitrate salt having copper2 as the couterion.
Are there not SIX MOLES OF OXYGEN ATOMS. Click to see full answer. Molecular weight of CuNO32 or mol This compound is also known as Copper Nitrate. One mole of. For each 1 CaNO32 there are six 2 NO3 or 2 3 O oxygens. Sboula sboula 10162017 Chemistry College answered expert verified How many moles of oxygen atoms are in 2 moles of Na3PO4.

What is the molecular formula of this compound. Moles of Mannose 214180 1889 Molecules of Mannose 1889 x avogadros number 6022 x 1023 716 x 1023 Number of Os in Mannose 6716 x 1023 4296 x 1023. How many moles of oxygen atoms are in 839 grams of potassium hydroxide. Is calcium a nitrate. How do I calculate moles.
 Source: studylib.net
Source: studylib.net
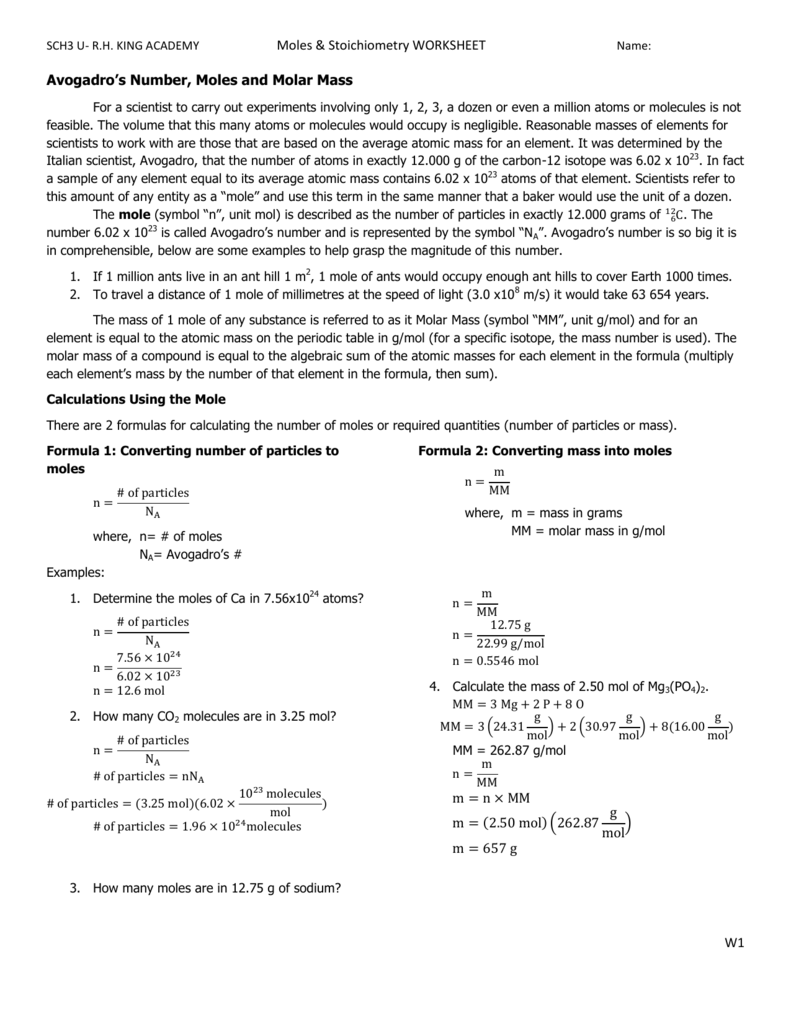
How many moles of oxygen are present. The number of molecules in 16 gm of oxygen are 05 1632 moles. Since the number of Oxygen atoms in a CO2 is doubled the number of Oxygen atoms in a CO. That is the molar mass of a substance is the mass in grams per mole of 6022 10 23 atoms molecules or formula units of that substance. You know that one mole of calcium carbonate contains 1 mole of calcium one mole of carbon and 3 moles of oxygen.
 Source: freeguruhelpline.com
Source: freeguruhelpline.com
Molecular weight of CuNO32 or mol This compound is also known as Copper Nitrate. How many moles of H atoms are in 34g of ammonia. You can view more details on each measurement unit. That is the molar mass of a substance is the mass in grams per mole of 6022 10 23 atoms molecules or formula units of that substance. The number of molecules in 16 gm of oxygen are 05 1632 moles.
 Source: clutchprep.com
Source: clutchprep.com
In one mole of NO2 you would have 1 mole of N and 2 moles of O. Since you have 2 oxygen atoms in one molecule there are 2 6022 10 23 O atoms in a mole of. Molecular weight of CuNo3 or grams The SI base unit for amount of substance is the mole. Sboula sboula 10162017 Chemistry College answered expert verified How many moles of oxygen atoms are in 2 moles of Na3PO4. Click to see full answer.
 Source: freeguruhelpline.com
Source: freeguruhelpline.com
Since the number of Oxygen atoms in a CO2 is doubled the number of Oxygen atoms in a CO. 1 grams CuNO32 is equal to 00053317466055435 mole. For each 1 CaNO32 there are six 2 NO3 or 2 3 O oxygens. CopperII nitrate CuNO32 is an inorganic compound that forms a blue crystalline solid. Is CU no3 2 a salt.
 Source: clutchprep.com
Source: clutchprep.com
How many moles of oxygen atoms are in 2 moles of Na3PO4. You can view more details on each measurement unit. How many moles of H atoms are in 34g of ammonia. Answered 2 years ago Author has 885 answers and 845K answer views. A compound contains by mass 267 carbon 711 oxygen and the remainder hydrogen.
 Source: youtube.com
Source: youtube.com
Weight of 1 mole of oxygen is 16 g and Al is 27 g. Add your answer and earn points. Weight of 1 mole of oxygen is 16 g and Al is 27 g. Answer and Explanation. You can view more details on each measurement unit.
 Source: 9.realinfo.tv
Source: 9.realinfo.tv
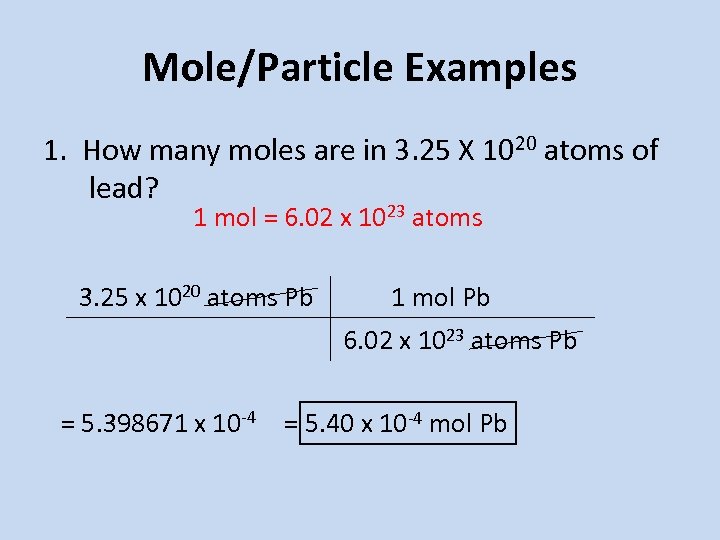
Weight of 1 mole of oxygen is 16 g and Al is 27 g. The number of molecules in 16 gm of oxygen are 05 1632 moles. 140 g x 1 mole18756 g 00746 moles00746 moles CuNO32 x 6 moles Omole CuNO32 0448 moles O atoms0448 moles O atoms x 602x1023 atomsmole 270x1023 atoms of oxygen. Molecular weight of CuNO32 or mol This compound is also known as Copper Nitrate. That is the molar mass of a substance is the mass in grams per mole of 6022 10 23 atoms molecules or formula units of that substance.
 Source: studylib.net
Source: studylib.net
Since the number of Oxygen atoms in a CO2 is doubled the number of Oxygen atoms in a CO. In other words 1 mole of oxygen would contain molecules. A mole is not short for a molecule. Avogadros number represents the number of units in one mole of any substance. Type in your own numbers in the form to convert the units.
 Source: slideplayer.com
Source: slideplayer.com
Please explain how you did it. Since you have 2 oxygen atoms in one molecule there are 2 6022 10 23 O atoms in a mole of. 4 rows of Atoms. Please explain how you did it. You can view more details on each measurement unit.
 Source: studylib.net
Source: studylib.net
Moles of Mannose 214180 1889 Molecules of Mannose 1889 x avogadros number 6022 x 1023 716 x 1023 Number of Os in Mannose 6716 x 1023 4296 x 1023. 148 1024 atoms Cu 11 1022 atoms Ag 1 mol 6022 1023 atoms -2 mol Ag Molar Mass. What is the molecular formula of this compound. And there are also 1mol of copper atoms and 2mol of. A compound contains by mass 267 carbon 711 oxygen and the remainder hydrogen.

Is CU no3 2 a salt. Answer in units of mol. The number of molecules in 16 gm of oxygen are 05 1632 moles. Then that means 1 g Carbon atom of CO2 would combine with 133 2 266 g of Oxygen. Sboula sboula 10162017 Chemistry College answered expert verified How many moles of oxygen atoms are in 2 moles of Na3PO4.
 Source: slideplayer.com
Source: slideplayer.com
Molecular weight of CuNO32 or mol This compound is also known as Copper Nitrate. NH3 - 1 mole 17gmol So 17g of NH3 contains 3 mole of H atoms So 34g NH3. Then that means 1 g Carbon atom of CO2 would combine with 133 2 266 g of Oxygen. A 023 mole sample of this compound weighs 207 g. A compound contains by mass 267 carbon 711 oxygen and the remainder hydrogen.
 Source: studylib.net
Source: studylib.net
Then that means 1 g Carbon atom of CO2 would combine with 133 2 266 g of Oxygen. In respect to this what is the gram formula mass of. 1 mol C 1201 g C or 1201 g C 1 mol C Converting between Mass and Number of Moles Converting between Mass and Number of Atoms Calculate the moles of carbon in. 1 grams CuNO32 is equal to 00053317466055435 mole. Type in your own numbers in the form to convert the units.
 Source: slideplayer.com
Source: slideplayer.com
A mole is not short for a molecule. How many moles of oxygen atoms are in 839 grams of potassium hydroxide. Type in your own numbers in the form to convert the units. That is the molar mass of a substance is the mass in grams per mole of 6022 10 23 atoms molecules or formula units of that substance. Thus the number of molecules in 16 gm or 05 mole of oxygen gas are 05 x 6023 x 1023 3012 x 1023 molecules.
This site is an open community for users to submit their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site serviceableness, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title how many moles of oxygen atoms are in 1 mole of cuno32 by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.