Your How many moles of calcium are in 100 g images are available. How many moles of calcium are in 100 g are a topic that is being searched for and liked by netizens now. You can Get the How many moles of calcium are in 100 g files here. Download all free vectors.
If you’re searching for how many moles of calcium are in 100 g pictures information related to the how many moles of calcium are in 100 g topic, you have visit the right blog. Our website always gives you suggestions for refferencing the highest quality video and picture content, please kindly surf and find more enlightening video content and images that match your interests.
How Many Moles Of Calcium Are In 100 G. Weigh about 2 g of calcium phosphate to the nearest 0001 g. The SI base unit for amount of substance is the mole. 6022 x 1023 02495134 moles 15025697 x 1022 atoms. Molecular weight of CaCl2 or mol This compound is also known as Calcium Chloride.
 Convert 100 Grams Of Calcium To Moles Of Calcium 100 G To Moles Of Ca From convertermaniacs.com
Convert 100 Grams Of Calcium To Moles Of Calcium 100 G To Moles Of Ca From convertermaniacs.com
Do a quick conversion. In order to find out how many atoms are in the sample multiply the amount is moles by using Avogadros number. How many atoms of oxygen are present in 300 grams of caco3. Molecular weight of Calcium or mol. 1 mole is equal to 1 moles Calcium Carbonate or 1000869 grams. How many moles of carbon are in CaCO3.
The molecular formula for Calcium is Ca.
How many grams are in 1 mole of calcium. 1 mole or 224 L CO2 at STP is obtained on thermal decomposition of 100 g of CaCO3 So 672L CO2 can be obtained from 30 g CaCO3. The SI base unit for amount of substance is the mole. Use this page to learn how to convert between moles Calcium Carbonate and gram. When calculating molecular weight of a chemical compound it tells us how many grams are in one mole of that substance. Use this web page to discover ways to convert between moles Calcium and gram.
 Source: numerade.com
Source: numerade.com
How many grams are in 1 mole of calcium. We assume you are converting between grams CaCl2 and mole. Calcium can be measured by the amount of the calcium salt mg of the cation plus the anion or mL of a specified concentration or the amount of elemental calcium in milligrams mg in milliequivalents mEq or in millimoles mmol. Molar mass of calcium carbonate 40 12 3 16 100 gmol. Therefore 100 mg of CaCO3 has 360221023100100000.
 Source: youtube.com
Source: youtube.com
100 g of Calcium Carbonate 100000 mg of Calcium Carbonate has 3 moles of Oxygen atoms 3 x 6022 x 1023 Oxygen atoms. The SI base unit for amount of substance is the mole. Use this web page to discover ways to convert between moles Calcium and gram. How many moles are in 25 grams. The SI base unit for amount of substance is the mole.

1 grams Calcium is equal to 0024951344877489 mole. The SI base unit for amount of substance is the mole. Solution Molecular mass of CaCO3 4012316 100 No. Molecular weight of Calcium or mol. Of oxygen atoms 15 60221023 90331023 atoms Mass of Oxygen atoms 15 16 24 g.
 Source: youtube.com
Source: youtube.com
1 mole is equal to 1 moles CaCO3 or 1000869 grams. 18066 1020 oxygen atoms. 1501023 atoms a10-23 atomsROUGH WORK. The total number of atoms in a substance can also be determined by using the relationship between grams moles and atoms. Calciums atomic mass is 40078 u now the given weight of the object is 1 g so divide by 40078 u.
 Source: scholr.com
Source: scholr.com
The SI base unit for amount of substance is the mole. 18066 1020 oxygen atoms. Weigh about 2 g of calcium phosphate to the nearest 0001 g. Mole of CaCO3 50g100g 05 05 mole of CaCO3 contains 15 moles of oxygen atoms No. The total number of atoms in a substance can also be determined by using the relationship between grams moles and atoms.
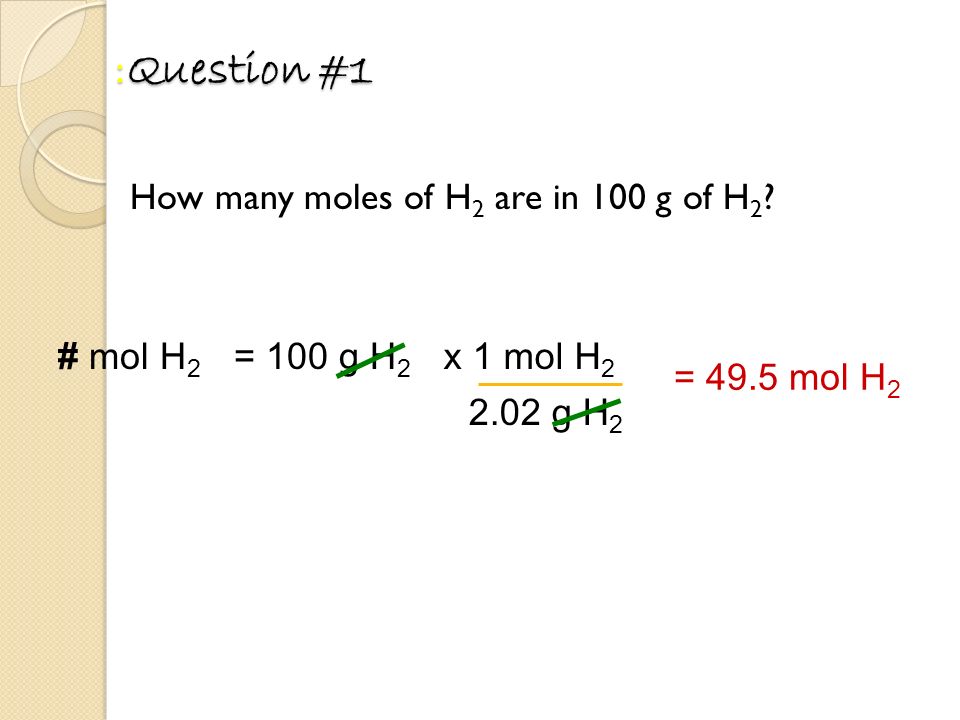 Source: slideplayer.com
Source: slideplayer.com
Calcium can be measured by the amount of the calcium salt mg of the cation plus the anion or mL of a specified concentration or the amount of elemental calcium in milligrams mg in milliequivalents mEq or in millimoles mmol. The formula for calcium phosphate is Ca 3PO 4 2. Finding molar mass starts with units of grams per mole gmol. Molecular mass of CaCO3 is 100g Ca40C12O163 100g CaCO3 100000 mg of CaCO3 has 3 moles of oxygen atoms 360221023 oxygen atoms. Molecular weight of Calcium or mol.
 Source: slideplayer.com
Source: slideplayer.com
How many moles of calcium carbonate CaCO3 are present in 20 g of the substance. What number of moles Calcium Phosphide in 1 grams. How many moles of carbon are in CaCO3. The SI base unit for amount of substance is the mole. As the sample contains 20 impuritiesit can be said that 80 g CaCO3 which will only produce CO2 is present in 100 g of sampleHence the initial mass of the sample should be 100X3080375 g.
 Source: pinterest.com
Source: pinterest.com
You can view more details on each measurement unit. Molecular mass of CaCO3 is 100g Ca40C12O163 100g CaCO3 100000 mg of CaCO3 has 3 moles of oxygen atoms 360221023 oxygen atoms. Molecular weight of Calcium or mol. Solution Molecular mass of CaCO3 4012316 100 No. Thus 1 mg of CaCO3 has 360221023100000 oxygen at.
 Source: convertermaniacs.com
Source: convertermaniacs.com
Because calcium has a valence of 2 the milliequivalents equals two times the number of. 6022 x 1023 02495134 moles 15025697 x 1022 atoms. The SI base unit for quantity of substance is the mole. 1501023 atoms a10-23 atomsROUGH WORK. 1 grams Calcium is equal to 0024951344877489 mole.
 Source: pinterest.com
Source: pinterest.com
1 mole is equal to 1 moles CaCO3 or 1000869 grams. Use this page to learn how to convert between moles Calcium Carbonate and gram. How many moles are in CaCO3. The formula for calcium phosphate is Ca 3PO 4 2. Of oxygen atoms 15 60221023 90331023 atoms Mass of Oxygen atoms 15 16 24 g.
 Source: brainly.in
Source: brainly.in
The formula for calcium phosphate is Ca 3PO 4 2. Weigh about 2 g of calcium phosphate to the nearest 0001 g. Because calcium has a valence of 2 the milliequivalents equals two times the number of. Use this page to learn how to convert between moles Calcium Carbonate and gram. 100 g of Calcium Carbonate 100000 mg of Calcium Carbonate has 3 moles of Oxygen atoms 3 x 6022 x 1023 Oxygen atoms.
 Source: pinterest.com
Source: pinterest.com
We assume you are converting between grams CaCl2 and mole. 1 mole or 224 L CO2 at STP is obtained on thermal decomposition of 100 g of CaCO3 So 672L CO2 can be obtained from 30 g CaCO3. 1 mole is equal to 1 moles CaCO3 or 1000869 grams. Mole of CaCO3 50g100g 05 05 mole of CaCO3 contains 15 moles of oxygen atoms No. Molecular weight of CaCl2 or mol This compound is also known as Calcium Chloride.
 Source: slideplayer.com
Source: slideplayer.com
The answer is 40078. You can view more details on each measurement unit. You know that one mole of calcium carbonate contains 1 mole of calcium one mole of. Solution Molecular mass of CaCO3 4012316 100 No. The molecular formula for Calcium is Ca.

The answer is 40078. Ca 40 C 12 O 16. We assume you are converting between grams Calcium and mole. Do a quick conversion. How many moles are in CaCO3.
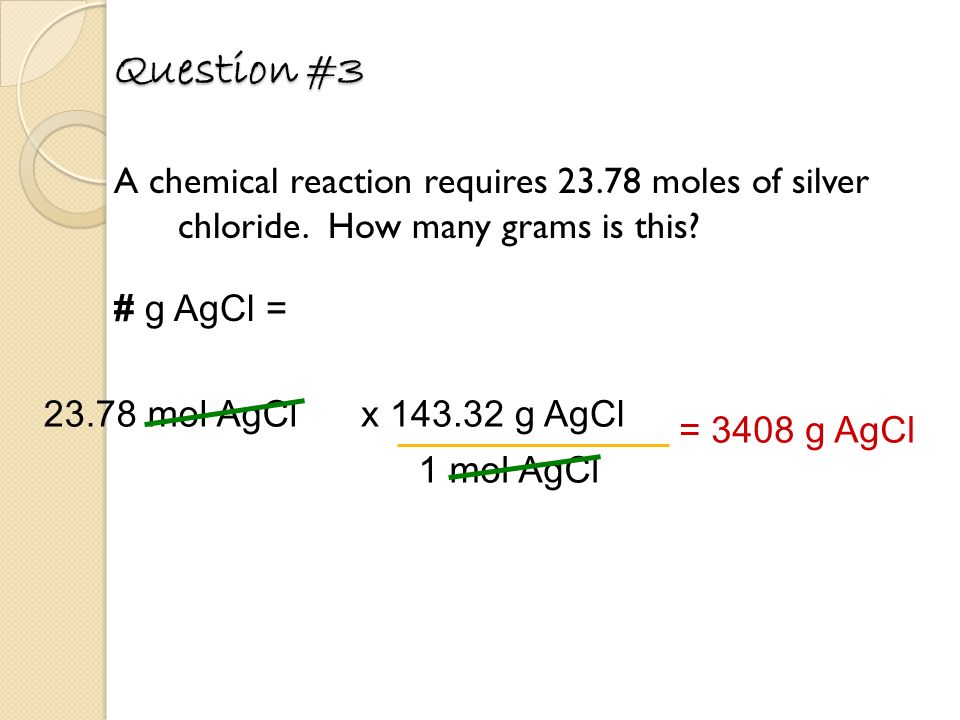 Source: slideplayer.com
Source: slideplayer.com
1 mole is equal to 1 moles CaCO3 or 1000869 grams. The molecular formula for Calcium is Ca. In one mole of MgCl 2 there are 2431 g of Mg molar mass of Mg the part we are interested in and 9521 g of MgCl 2 the whole thing so Mg in MgCl 2 is 24319521 x 100 2553 Mg 7 PROCEDURE Work individually. Of moles of CaCO₃ 200 g 1 mol 100 g 2 mol. 1501023 atoms a10-23 atomsROUGH WORK.
This site is an open community for users to do sharing their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site helpful, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title how many moles of calcium are in 100 g by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






