Your How many moles in 1 gram of nitrogen images are available in this site. How many moles in 1 gram of nitrogen are a topic that is being searched for and liked by netizens today. You can Get the How many moles in 1 gram of nitrogen files here. Find and Download all free photos.
If you’re looking for how many moles in 1 gram of nitrogen images information related to the how many moles in 1 gram of nitrogen keyword, you have come to the right site. Our site always gives you hints for downloading the highest quality video and picture content, please kindly hunt and find more enlightening video content and graphics that match your interests.
How Many Moles In 1 Gram Of Nitrogen. 1 grams CaBr2 is equal to 00050028516254265 mole. How many atoms are in 2 moles of ammonia. Question 1 Moles of nitrogen dioxide 225 mol Molecular formula of nitrogen dioxide NO2 So each mole of NO2 has 1 mole of nitrogen and 2 moles of oxygen. To start you need know that all a mole means is you have 6022 x 10 23 atoms of that type.
 Calculate No Of Molecules And No Of Atoms Present In 1 G Of Nitrogen From toppr.com
Calculate No Of Molecules And No Of Atoms Present In 1 G Of Nitrogen From toppr.com
Beside above how many atoms are in 15 moles of nitrogen. What is the mass of one mole of nh4 2 co3. This means that you must have. Therefore 2 moles will have 12041024 molecules of NH3. Note that rounding errors may occur so always check the results. Moles mol x Molar Mass gmol 1 x 5844.
Admin Send an email 4 weeks ago.
Boiling Point BP Nitrogen changes its state from liquid to gas at -19579C -320422F or 7736K Nitrogen gas is a diamagnetic colorless odorless and tasteless gas. But we want 1g how many molecules. How many moles of nitrogen are in 4561 gram of nitrogen. CAS Registry Number CAS RN. See more articles in category. Question 15 How many moles of nitrogen atoms are there in 164 x 1023 molecules of dinitrogen trichloride.
 Source: in.pinterest.com
Source: in.pinterest.com
The SI base unit for amount of substance is the mole. Note that rounding errors may occur so always check the results. Mass of nitrogen molecule in kg. N given mass molar mass n g i v e n m a s s m o l a r m a s s. In this manner how many atoms are in a mole.
 Source: pinterest.com
Source: pinterest.com
How many moles are in 25 grams of NH3. The number of moles is found as. P V nRT n P V RT. How many atoms are in 2 moles of ammonia. 0545 mol 0817 mol X 0272 mol 366 mol 110 mol Question 16 How many grams of chlorine atoms are present in a 317 g sample of barium chloride BaCl2.
 Source: pinterest.com
Source: pinterest.com
How many moles of nitrogen are in 4561 gram of nitrogen. 225 moles of NO2 will have 225 moles of nitrogen and 2 x 225 450 mol o View the full answer. For instance look at N Nitrogen you will see the atomic mass is 1400674 grams. See more articles in category. Note that rounding errors may occur so always check the results.
 Source: pinterest.com
Source: pinterest.com
Question 1 Moles of nitrogen dioxide 225 mol Molecular formula of nitrogen dioxide NO2 So each mole of NO2 has 1 mole of nitrogen and 2 moles of oxygen. How much does 1 mole of Nitrogen weigh in grams. So that means if you had 1 mole of nitrogen atoms it would weight 1400674 grams. Its molecular mass will become 28. NN 2 99707 101325 atm 75L 0082 atm L molK 27315 25K 302 moles N2.
 Source: toppr.com
Source: toppr.com
Nitrogen gas is defined as N2. The molar mass of nitrogen n2 is 280 gmole what is the mass of a single nitrogen atom 1 mole of nitrogen gas. Mass of nitrogen molecule in kg. Its molecular mass will become 28. The SI base unit for amount of substance is the mole.
 Source: pinterest.com
Source: pinterest.com
If you want you can just read the first method for finding the second part of the question but the second method will be useful to you in future if you are not already. CAS Registry Number CAS RN. The number of moles is found as. Enter your answer in decimal form with the correct number of sig figs. To start you need know that all a mole means is you have 6022 x 10 23 atoms of that type.
 Source: toppr.com
Source: toppr.com
How much does 1 mole of Nitrogen weigh in grams. Mass of nitrogen gas in grams. 5 1 4 7 g r a m 2 1 m o l e 2 1 6. Divide given mass in gram of substance with its molecular mass the number you get is number of moles. Note that rounding errors may occur so always check the results.

The molecular formula for Nitrogen is N. This means that you must have. There are 411022 nitrogen atoms in 150g and 1201024 in a 440g sample. Use the ideal gas law equation to determine how many moles of nitrogen were produced. Mass of nitrogen molecule in kg.
 Source: toppr.com
Source: toppr.com
4 moles of nitrogen atoms is equal to 2 moles of nitrogen the molecule is diatomic - N2. Question 1 Moles of nitrogen dioxide 225 mol Molecular formula of nitrogen dioxide NO2 So each mole of NO2 has 1 mole of nitrogen and 2 moles of oxygen. According to the mole concept in one mole there is 60221023 moles are presentso mass of one molecule of nitrogen gas 2860221023. Admin Send an email 4 weeks ago. So that means if you had 1 mole of nitrogen atoms it would weight 1400674 grams.
 Source: toppr.com
Source: toppr.com
The molar mass of nitrogen n2 is 280 gmole what is the mass of a single nitrogen atom 1 mole of nitrogen gas. Enter your answer in decimal form with the correct number of sig figs. If you want you can just read the first method for finding the second part of the question but the second method will be useful to you in future if you are not already. Mass of nitrogen molecule in kg. The molecular formula for Nitrogen is N.
 Source: youtube.com
Source: youtube.com
If we divide both sides of the equation by the number 28 we get 28g28 202 x1023molecules28. One mole of anything is defined as its molecular weight in grams. Note that rounding errors may occur so always check the results. So that means if you had 1 mole of nitrogen atoms it would weight 1400674 grams. How many moles of nitrogen are in 4561 gram of nitrogen.
 Source: pinterest.com
Source: pinterest.com
1 10 -6. So that means if you had 1 mole of nitrogen atoms it would weight 1400674 grams. Nitrogen gas is defined as N2. What is the mass of one mole of nh4 2 co3. NN 2 99707 101325 atm 75L 0082 atm L molK 27315 25K 302 moles N2.
 Source: youtube.com
Source: youtube.com
A mole of a substance is referred to the mass of a substance containing the same number of fundamental units as there are atoms in exactly 12000 g. The molar mass of nitrogen n2 is 280 gmole what is the mass of a single nitrogen atom 1 mole of nitrogen gas. Nitrogen N Molecular weight. Mass of nitrogen molecule in kg. Mass of nitrogen 14 g.
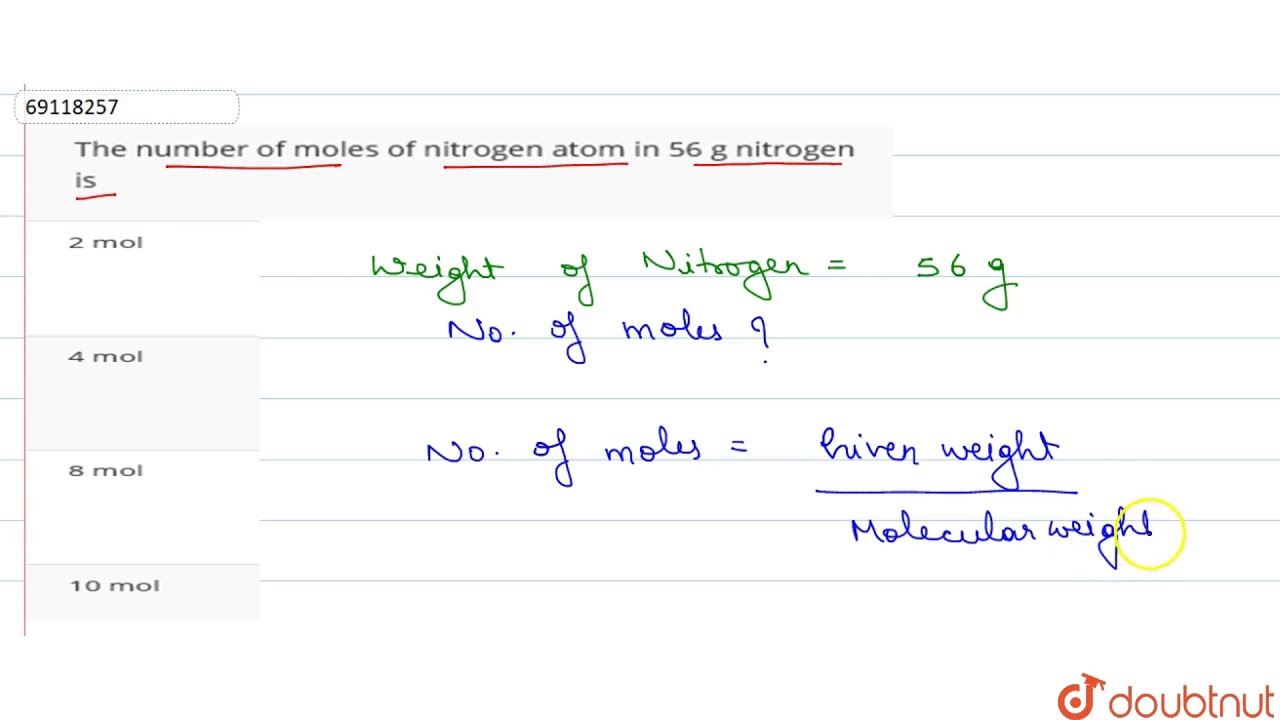 Source: youtube.com
Source: youtube.com
In this manner how many atoms are in a mole. CAS Registry Number CAS RN. You know that the reaction produced enough moles to occupy 75 L at those conditions of pressure and temperature. So that means if you had 1 mole of nitrogen atoms it would weight 1400674 grams. So that means if you had 1 mole of nitrogen atoms it would weight 1400674 grams.
 Source: youtube.com
Source: youtube.com
How many atoms are in 2 moles of ammonia. Molecules NH3 147 moles x 6021023 moleculesmole 891023 molecules of NH3 to 2 sig. According to the mole concept in one mole there is 60221023 moles are presentso mass of one molecule of nitrogen gas 2860221023. Boiling Point BP Nitrogen changes its state from liquid to gas at -19579C -320422F or 7736K Nitrogen gas is a diamagnetic colorless odorless and tasteless gas. 350 moles nitrogen 1 mole N1401 grams 250 moles nitrogen—————————-.
This site is an open community for users to submit their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site helpful, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title how many moles in 1 gram of nitrogen by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






