Your How many moles in 1 gram of carbon dioxide images are ready in this website. How many moles in 1 gram of carbon dioxide are a topic that is being searched for and liked by netizens now. You can Get the How many moles in 1 gram of carbon dioxide files here. Get all free images.
If you’re looking for how many moles in 1 gram of carbon dioxide pictures information related to the how many moles in 1 gram of carbon dioxide keyword, you have pay a visit to the ideal blog. Our website always provides you with hints for seeking the highest quality video and image content, please kindly surf and find more enlightening video articles and images that fit your interests.
How Many Moles In 1 Gram Of Carbon Dioxide. 44 grams is a mole of carbon dioxide and there is one carbon atom in CO 2 so there is one mole of carbon. So we have to find out how many moles there are in 22g of CO2. 1 grams Carbon Dioxide is equal to 0022722366761722 mole. The number ofmoles is 664415.

The molar mass of carbon and oxygen is. 1 120 2 160 440 gmol. The molecular formula for Carbon Dioxide is CO2. Therefore there willbe 0044643 moles 1224 here. Carbon dioxide weighs 0001836 gram per cubic centimeter or 1836 kilogram per cubic meter ie. The number ofmoles is 664415.
M 140 mol440 gmol.
At 25C 77F or 29815K at standard atmospheric pressure. The SI base unit for amount of substance is the mole. 1 mole is equal to 1 moles CO2 or 440095 grams. Now we have the molecular weight of the compound and the given mass of compound which is 28 g. 06 g of carbon dioxide. Carbon dioxide weighs 0001836 gram per cubic centimeter or 1836 kilogram per cubic meter ie.

1 10 -6. 06 g of carbon dioxide. The weight of CO2 is 44 grams per mole 1 x 12 gramsmole for the carbon and 2 x 16 gramsmole for the oxygen atoms. 6 How many molecules are in carbon dioxide. For our practice problem well convert 25.

There are 6022 X 1023 molecules of CO2 in a mole enough to make the grams in the mole equal the mass. This tells you how many atoms you have in one mole. Since there are two atoms of oxygen in CO 2 that means there are two moles of oxygen atoms in 44 grams. Is the mass of one molecule of CO2 while 4401 g is the mass of one mole of CO2 or 6022 x 1023 molecules of CO2 or 3 x 602 x 1023 total number of atoms. Density of carbon dioxide is equal to 1836 kgm³.

How many moles are there in 52. New questions in Chemistry. Mass 22g molar mass relative atomic mass of carbon 12 twice the relative atomic mass of oxygen 16 12 2 x 16 44. 44 grams is a mole of carbon dioxide and there is one carbon atom in CO 2 so there is one mole of carbon. Environment Canada publishes factors to estimate CO2.
 Source: slideplayer.com
Source: slideplayer.com
6 How many molecules are in carbon dioxide. The weight of CO2 is 44 grams per mole 1 x 12 gramsmole for the carbon and 2 x 16 gramsmole for the oxygen atoms. The mass of the carbon dioxide is determined from the definition of molar mass. 1 10 -6. 28g CO2 x 1 mol CO244g CO2 0636 mol of CO2.
 Source: brainly.in
Source: brainly.in
We have to convert moles to mass of carbon dioxide. 440 1783 7845 grams. So we have to find out how many moles there are in 22g of CO2. Note that rounding errors may occur so always check the results. Mr of carbon dioxide is 2161244 Now times by Abogadros constant.

You can view more details on each measurement unit. M m n m n M M m n m n M. Molecular weight of Carbon Dioxide or mol. 1 grams CO2 is equal to 0022722366761722 mole. Now we have the molecular weight of the compound and the given mass of compound which is 28 g.
 Source: clutchprep.com
Source: clutchprep.com
Density of carbon dioxide is equal to 1836 kgm³. In chemistry the molar mass of a chemical compound is defined as the mass of a sample of that compound divided by the amount of substance in that sample measured in moles. Therefore there willbe 0044643 moles 1224 here. According to avogadros law 1 mole of every substance occupies 224 L at STP and contains avogadros number of particles. This number is known as Avogadro.
 Source: slideplayer.com
Source: slideplayer.com
000106128 ounce oz of Carbon dioxide fits into 1 cubic inch. 1 grams Carbon Dioxide is equal to 0022722366761722 mole. Is the mass of one molecule of CO2 while 4401 g is the mass of one mole of CO2 or 6022 x 1023 molecules of CO2 or 3 x 602 x 1023 total number of atoms. 1 moles Carbon Dioxide 440095 gram using the molecular weight calculator and the molar mass of CO2. Is the mass of one molecule of CO2 while 4401 g is the mass of one mole of CO2 or 6022 x 1023 molecules of CO2 or 3 x 602 x 1023 total number of atoms.
 Source: slideplayer.com
Source: slideplayer.com
Since there are two atoms of oxygen in CO 2 that means there are two moles of oxygen atoms in 44 grams. This number is known as Avogadro. At STP 1 mole will 224 litres. 1836 kilograms kg of Carbon dioxide fit into 1 cubic meter. There are 6022 X 1023 molecules of CO2 in a mole enough to make the grams in the mole equal the mass.
 Source: toppr.com
Source: toppr.com
Finding out the molar mass of. Which gives more CO2 per gram fuel. 44 grams is a mole of carbon dioxide and there is one carbon atom in CO 2 so there is one mole of carbon. The number of atoms of carbon-12 present in this one mole sample is 6022 136 7 x 10 23. Molecular weight of Carbon Dioxide or mol.
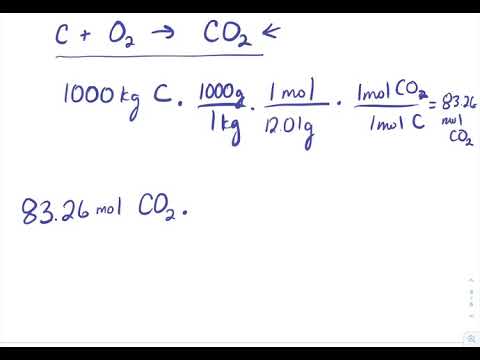 Source: chem.libretexts.org
Source: chem.libretexts.org
O 160 gmol. The SI base unit for amount of substance is the mole. 28g CO2 x 1 mol CO244g CO2 0636 mol of CO2. The mass of the carbon dioxide is determined from the definition of molar mass. The mass of gas you gave is 66 g.

We use the molar mass of carbon dioxide to convert moles to mass of. 44 grams is a mole of carbon dioxide and there is one carbon atom in CO 2 so there is one mole of carbon. Now we have the molecular weight of the compound and the given mass of compound which is 28 g. 1 120 2 160 440 gmol. 6 How many molecules are in carbon dioxide.
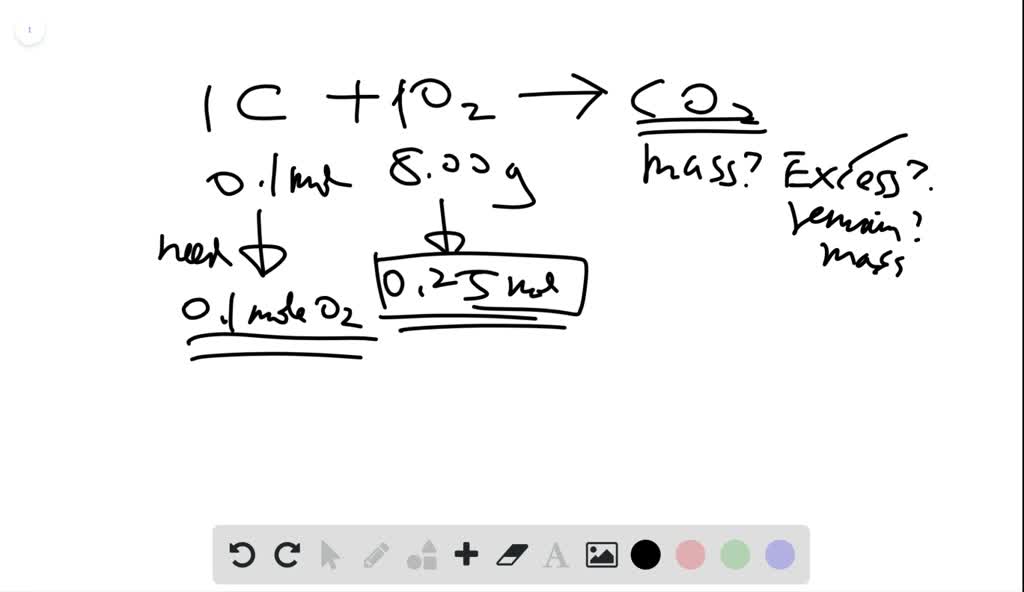 Source: numerade.com
Source: numerade.com
M m n m n M M m n m n M. You can view more details on each measurement unit. So 3 moles of CO2 contains 3 x 2 x 602 x 1023 O atoms 361 x 1024 O atoms. This compound is also known as Carbon Dioxide. The mass of CO2 is.
 Source: slideplayer.com
Source: slideplayer.com
Have a nice day. There are 6022 X 1023 molecules of CO2 in a mole enough to make the grams in the mole equal the mass. 440 1783 7845 grams. 6 How many molecules are in carbon dioxide. The SI base unit for amount of substance is the mole.

Which gives more CO2 per gram fuel. The answer is 52063238500000004. 6 How many molecules are in carbon dioxide. Therefore there willbe 0044643 moles 1224 here. Density of carbon dioxide is equal to 1.
This site is an open community for users to do sharing their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site good, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title how many moles in 1 gram of carbon dioxide by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






