Your How many moles are there in 100 grams of carbon dioxide images are available in this site. How many moles are there in 100 grams of carbon dioxide are a topic that is being searched for and liked by netizens today. You can Find and Download the How many moles are there in 100 grams of carbon dioxide files here. Download all royalty-free images.
If you’re looking for how many moles are there in 100 grams of carbon dioxide pictures information related to the how many moles are there in 100 grams of carbon dioxide topic, you have come to the ideal site. Our site always provides you with suggestions for refferencing the highest quality video and picture content, please kindly hunt and locate more informative video articles and graphics that fit your interests.
How Many Moles Are There In 100 Grams Of Carbon Dioxide. Note that rounding errors may occur so always check the results. Also Know how many atoms are there in one mole of carbon. How many moons are there in 22 gram of carbon dioxide. So 3 moles of CO2 contains 3 x 2 x 602 x 1023 O atoms 361 x 1024 O atoms.

1 mole of carbon. 1 mole is equal to 1 moles CO2 or 440095 grams. The mass of CO2 is. 833 moles CO2 X. Calculate the volume of carbon dioxide gas produced at STP if 1000 grams of glucose reacts. 100 grams of carbon dioxide contain 227 moles.
Converting from grams of elements to moles.
199 mg H2O X. Express mass as grams. The SI base unit for amount of substance is the mole. We assume you are converting between grams Carbon Dioxide and mole. The number of atoms of carbon-12 present in this one mole sample is 6022 136 7 x 10 23. So 3 moles of CO2 contains 3 x 2 x 602 x 1023 O atoms 361 x 1024 O atoms.
 Source: slideplayer.com
Source: slideplayer.com
Consequently how many moles of co2 are in the atmosphere. Since there are two atoms of oxygen in CO_2 that means there are two moles of oxygen. Calculate the volume of carbon dioxide gas produced at STP if 1000 grams of glucose reacts. 44 grams is a mole of carbon dioxide and there is one carbon atom in CO_2 so there is one mole of carbon. 40 grams Carbon Dioxide to mol 090889 mol.
 Source: youtube.com
Source: youtube.com
There are 05 moles in 22g of Carbon Dioxide. Exactly 12 grams of pure carbon-12 powder is known as one mole. Molecular weight of CO2 or grams This compound is also known as Carbon Dioxide. The SI base unit for amount of substance is the mole. Molecular weight of CO2 or mol This compound is also known as Carbon Dioxide.

What is the volume in liters of a 300 mole sample of carbon dioxide at STP. As youve learned the concentration of carbon dioxide CO2 in the atmosphere is now 400 parts per million. Must be used to produce 199 grams of water. Since there are two atoms of oxygen in CO_2 that means there are two moles of oxygen. Mass moles x RAM mass 5 67 x 44 249 48 grams of carbon dioxide.
 Source: clutchprep.com
Source: clutchprep.com
Consequently how many moles of co2 are in the atmosphere. 200 grams Carbon Dioxide to mol 454447 mol. Now in mol of CO2 there are 6022x10 23 molecules and in each molecule there are 2 atoms of O. Calcium carbonate decomposes at high temperature forming calcium oxide and carbon dioxide according to the following reaction. The molecular formula for Carbon Dioxide is CO2.
 Source: slideplayer.com
Source: slideplayer.com
44 grams is a mole of carbon dioxide and there is one carbon atom in CO_2 so there is one mole of carbon. Mass moles x RAM mass 5 67 x 44 249 48 grams of carbon dioxide. 44 grams is a mole of carbon dioxide and there is one carbon atom in CO_2 so there is one mole of carbon. 250L x 1 mol224 L 0112 moles CO 2. Convert grams to moles and find the empirical formula.

833 moles CO2 X. How many moles of C. How many moles of carbon dioxide are there in 19 grams of carbon. The SI base unit for amount of substance is the mole. Note that rounding errors may occur so always check the results.

CaCO 3 s CaO s CO 2 g If 100 kg of CaCO 3 is decomposed by this reaction how many kilograms. 1 mole is equal to 1 moles Carbon Dioxide or 440095 grams. Molecular weight of CO2 or mol This compound is also known as Carbon Dioxide. This number is known as Avogadro. Similarly there are 671 g H and 533 g O.

The SI base unit for amount of substance is the mole. How many moles of carbon dioxide are contained in 100 grams of carbon dioxide. Since there are two moles of CO2 we can produce 2 6 1 1 3 moles of glucose C6H 12O6. 833 moles CO2 X. Molecular weight of CO2 or mol This compound is also known as Carbon Dioxide.
 Source: chegg.com
Source: chegg.com
Since there are two moles of CO2 we can produce 2 6 1 1 3 moles of glucose C6H 12O6. The SI base unit for amount of substance is the mole. 200 grams Carbon Dioxide to mol 454447 mol. If we start with 250 moles of butane how many moles of water will form. The answer is 440095.
 Source: toppr.com
Source: toppr.com
1mol H2O 180g X 2mol C8H18 18mol H2O 123 x 10-2 mol C8H18 Answer _____ c. 100 grams of carbon dioxide contain 227 moles. 40 grams Carbon Dioxide to mol 090889 mol. Convert grams to moles and find the empirical formula. Thats how chemistry works.

2 130 moles O. If 175 moles of ethanol were produced how many moles of glucose were there in the beginning. So here there exist. How many moles of CO2 are there in 1000 grams of CO2. The mass of CO2 is.
 Source: youtube.com
Source: youtube.com
Molecular weight of CO2 or mol This compound is also known as Carbon Dioxide. 1 mole is equal to 1 moles CO2 or 440095 grams. 1783 moles has been provided for you. Mass moles x RAM mass 5 67 x 44 249 48 grams of carbon dioxide. Carbon dioxide has a molar mass of 44 gmol.
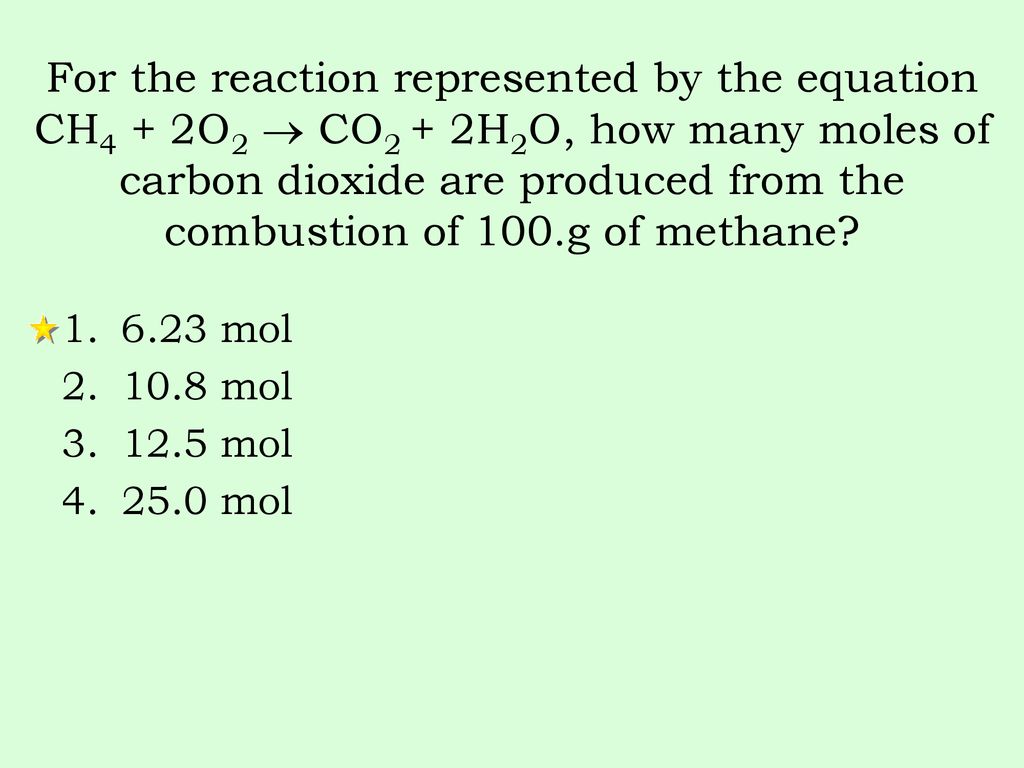 Source: slideplayer.com
Source: slideplayer.com
The total number of moles of gas in the atmosphere is 18 x 1020. Number of moles mass of substance molar mass. Note that rounding errors may occur so always check the results. 199 mg H2O X. 1 mole is equal to 1 moles Carbon Dioxide or 440095 grams.
 Source: chegg.com
Source: chegg.com
The number of atoms of carbon-12 present in this one mole sample is 6022 136 7 x 10 23. 100 grams of carbon dioxide contain 227 moles. The SI base unit for amount of substance is the mole. How many moons are there in 22 gram of carbon dioxide. The mass of CO2 is.
 Source: slideplayer.com
Source: slideplayer.com
How many grams Carbon Dioxide in 1 mol. The SI base unit for amount of substance is the mole. 2 130 moles O. Number of moles mass of substance molar mass. 199 mg H2O X.
This site is an open community for users to do submittion their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site helpful, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title how many moles are there in 100 grams of carbon dioxide by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.