Your How many moles are in 8 grams of ch4 images are available. How many moles are in 8 grams of ch4 are a topic that is being searched for and liked by netizens now. You can Find and Download the How many moles are in 8 grams of ch4 files here. Download all royalty-free photos and vectors.
If you’re looking for how many moles are in 8 grams of ch4 pictures information related to the how many moles are in 8 grams of ch4 interest, you have visit the ideal site. Our website always provides you with suggestions for seeing the highest quality video and picture content, please kindly search and locate more informative video content and images that fit your interests.
How Many Moles Are In 8 Grams Of Ch4. 1 moles CH4 to grams 1604246 grams. Firstly we need to know that Avogadros number 6023 1023 is the number of atoms molecules or groups of ions contained within one mole of a substance. The 05 moles of CH4 would normally consume 10 moles of O2. 1 mole is equal to 1 moles CH4 or 1604246 grams.
 How To Convert Moles Of Ch4 To Grams Youtube From youtube.com
How To Convert Moles Of Ch4 To Grams Youtube From youtube.com
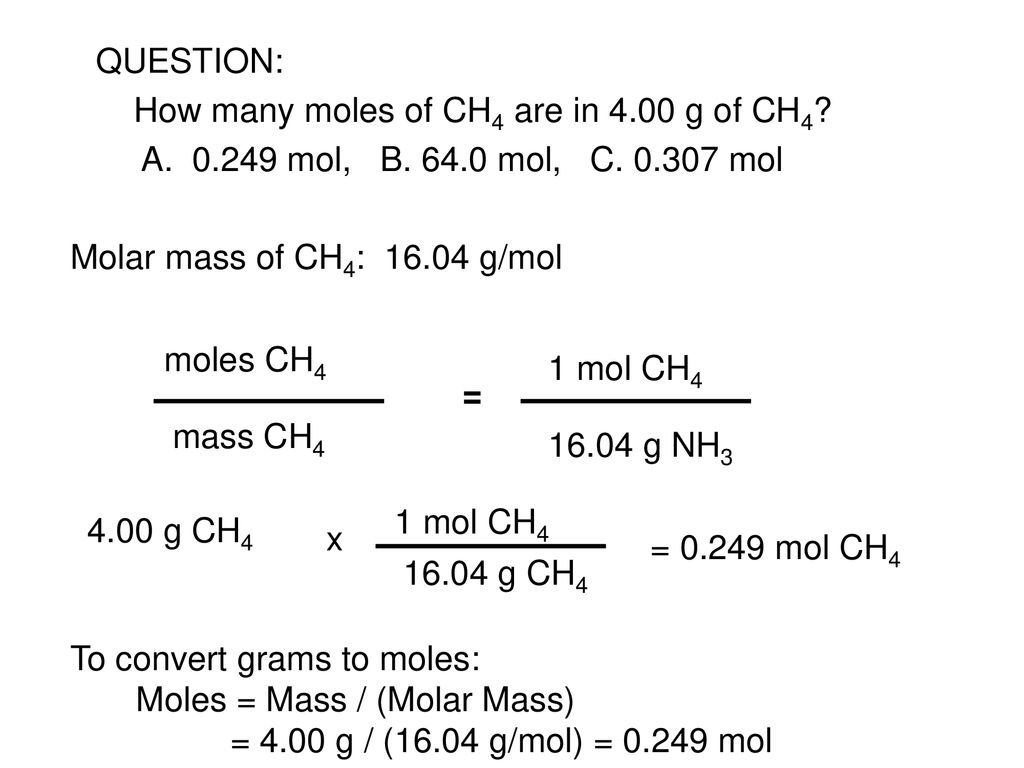
The technique used can be applied to any mole to gram conversion. C 120107 H4 100794 x 4 403176 total 1604246g 2. How many molecules are there in 216 grams of CH4. Molar mass CH4 16gmol. Determination of the mass of CH4. Find the moles of CH4 by dividing its mass by its molecular mass.
Number of mole CH4 0347 mole.
Find the moles of CH4 by dividing its mass by its molecular mass. In your case a mole of methane represents a collection of methane molecules. 8 moles CH4 to grams 12833968 grams. 8 If 100 grams of H 2 are mixed with 750 grams of O2 which reactant is the limiting reagent. The technique used can be applied to any mole to gram conversion. 1 grams CH4 is equal to 0062334579609362 mole.
 Source: pinterest.com
Source: pinterest.com
How many molecules are there in 216 grams of CH4. We assume you are converting between grams CH4 and mole. 8 g CH4 16 gmole 050 mole of CH4. The mass of CH4 can be obtained as follow. Now once you calculate you should get 71725g.
 Source: toppr.com
Source: toppr.com
We assume you are converting between grams CH4 and mole. 2 H 2 g O 2 g 2 H 2O l Limiting Reactants Chapter 4 Step 1. Convert mass to moles Moles H 2 100 g H 2 x 1 mole 495 mol H 2 available 202 g Moles O 2 750 g O 2x 1 mole 234 mol O 2 available 320 g Method 1 Limiting Reactants Chapter 4. Use this page to learn how to convert between grams CH4 and mole. Methane CH4 C 12 grams per mole H 1 gram per mole so CH4 16 gramsmole of which 4 are H so 4 grams.
 Source: toppr.com
Source: toppr.com
Moles of CH₄ 63 Further explanationGiven38 x 10²⁴ molecules of CH₄ gasRequiredThe number of molesSolution1 mol 602 x 10²³ particles molecules atoms. Molar mass CH4 16gmol. The technique used can be applied to any mole to gram conversion. Find the total mass of CH4. This means that O2 is the limiting reagent.
 Source: brainly.in
Source: brainly.in
In the above equation how many grams of water can be made when 18 moles of HNO3 are consumed. This means that O2 is the limiting reagent. Use this page to learn how to convert between grams CH4 and mole. In this video well learn to convert moles of CH4 to grams. We assume you are converting between grams CH4 and mole.
 Source: youtube.com
Source: youtube.com
05 mole of CH4 2 x 05mol 1 mol. Molar mass CH4 16gmol. 6 g O2 32 gmole 01875 mole of O2. 4 moles CH4 to grams 6416984 grams. The 05 moles of CH4 would normally consume 10 moles of O2.
 Source: brainly.in
Source: brainly.in
560 moles CH4 4 moles H1 mole CH41008 grams1 mole H 226 grams of hydrogen. The technique used can be applied to any mole to gram conversion. Whats The approach used might be utilized to any mole to. Find the moles of CH4 by dividing its mass by its molecular mass. We assume you are converting between moles CH4 and gram.
 Source: toppr.com
Source: toppr.com
First determine molar mass of CH4C12gmol 4x H1gmol16gmolThen divide by the number of grams64g16gmol 4 moles of CH4. You can view more details on each measurement unit. 8 g CH4 16 gmole 050 mole of CH4. 162 For the reaction C 2H2 CH4 how many grams of hydrogen are required to produce 54 moles of methane CH4. Note that this calculation is consistent dimensionally.
 Source: numerade.com
Source: numerade.com
To find how many molecules are present in 58. 8 g CH4 16 gmole 050 mole of CH4. Convert mass to moles Moles H 2 100 g H 2 x 1 mole 495 mol H 2 available 202 g Moles O 2 750 g O 2x 1 mole 234 mol O 2 available 320 g Method 1 Limiting Reactants Chapter 4. You can view more details on each measurement unit. In the above equation how many grams of water can be made when 18 moles of HNO3 are consumed.
 Source: topperlearning.com
Source: topperlearning.com
How many moles CH4 in 1 grams. This means that O2 is the limiting reagent. 05 mole of CH4 2 x 05mol 1 mol. In your case a mole of methane represents a collection of methane molecules. You can view more details on each measurement unit.
 Source: brainly.in
Source: brainly.in
N2 moles CH4 Since methane CH4 contains 4 hydrogen atoms per mole then 2 moles of methane would mean there are 8 moles of hydrogen 2 x 4 8 Then multiply the amount of moles of hydrogen by Avogadros number. 602210²³ molecules of CH4 which consists of 1 mole of C atoms and 4 moles of H atoms. How many molecules are there in 216 grams of CH4. Molar mass CH4 16gmol. So from this we know there are 6023 1023 molecules in one mole of CH4.
 Source: youtube.com
Source: youtube.com
To find the number of moles all we have to do is use the following relationship. You can always use our grams to moles calculator to check the result. What number of moles are in 8 grams of ch4. 1 moles CH4 to grams 1604246 grams. Find the moles of CH4 by dividing its mass by its molecular mass.
 Source: topperlearning.com
Source: topperlearning.com
05 mole of CH4 2 x 05mol 1 mol. What number of moles are in 8 grams of ch4. Note that this calculation is consistent dimensionally. N2 moles CH4 Since methane CH4 contains 4 hydrogen atoms per mole then 2 moles of methane would mean there are 8 moles of hydrogen 2 x 4 8 Then multiply the amount of moles of hydrogen by Avogadros number. In the above equation how many grams of water can be made when 18 moles of HNO3 are consumed.
 Source: pinterest.com
Source: pinterest.com
8 If 100 grams of H 2 are mixed with 750 grams of O2 which reactant is the limiting reagent. More specifically a mole of methane contains 60221023 molecules of methane this is known as Avogadros number. 2 moles CH4 to grams 3208492 grams. How many molecules are there in 216 grams of CH4. Moles of CH₄ 63 Further explanationGiven38 x 10²⁴ molecules of CH₄ gasRequiredThe number of molesSolution1 mol 602 x 10²³ particles molecules atoms.
 Source: youtube.com
Source: youtube.com
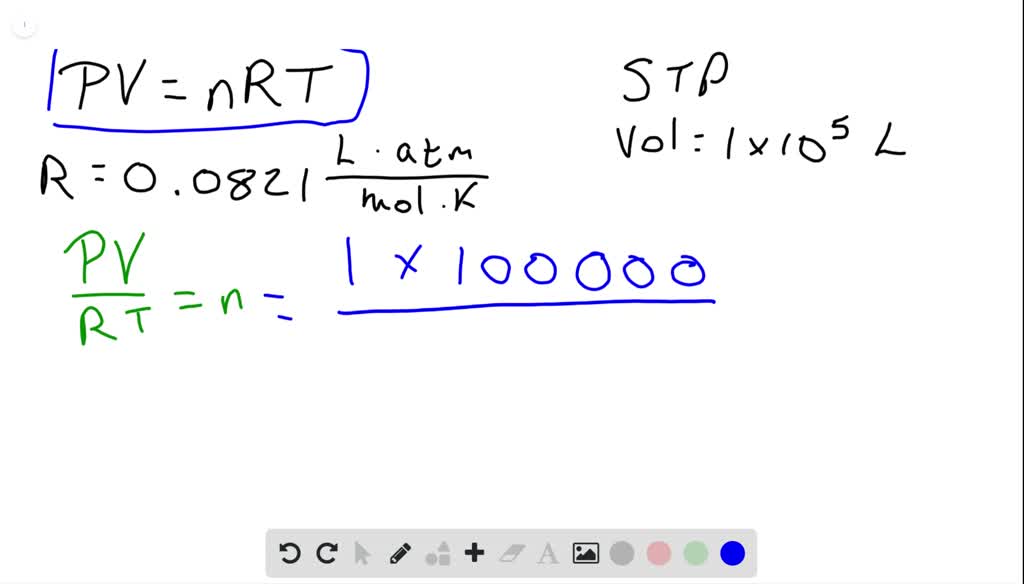
N 2783 x 875 8314 x 843 n 0347 mole. We assume you are converting between moles CH4 and gram. Molar mass CH4 16gmol. 4 moles CH4 to grams 6416984 grams. Number of mole CH4 0347 mole.
 Source: slideplayer.com
Source: slideplayer.com
We assume you are converting between grams CH4 and mole. Compare the mole ratios. So from this we know there are 6023 1023 molecules in one mole of CH4. N 2783 x 875 8314 x 843 n 0347 mole. You can always use our grams to moles calculator to check the result.
This site is an open community for users to do sharing their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site helpful, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title how many moles are in 8 grams of ch4 by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






