Your How many moles are in 5 grams of calcium chloride images are available in this site. How many moles are in 5 grams of calcium chloride are a topic that is being searched for and liked by netizens now. You can Download the How many moles are in 5 grams of calcium chloride files here. Download all free vectors.
If you’re looking for how many moles are in 5 grams of calcium chloride images information connected with to the how many moles are in 5 grams of calcium chloride interest, you have visit the ideal site. Our site always provides you with hints for downloading the highest quality video and image content, please kindly surf and locate more enlightening video content and graphics that fit your interests.
How Many Moles Are In 5 Grams Of Calcium Chloride. 1 mole calcium chloride 402355 111gmol. Molar mass of NaCl is 58443 how many grams is 5 mole NaCl. The answer of How many moles are in 222 grams of Calcium chloride CaCl2. To calculate the moles we use the equation.
 Pin By Threefourthsme On Chemistry Covalent Bonding Teaching Style Classwork From cz.pinterest.com
Pin By Threefourthsme On Chemistry Covalent Bonding Teaching Style Classwork From cz.pinterest.com
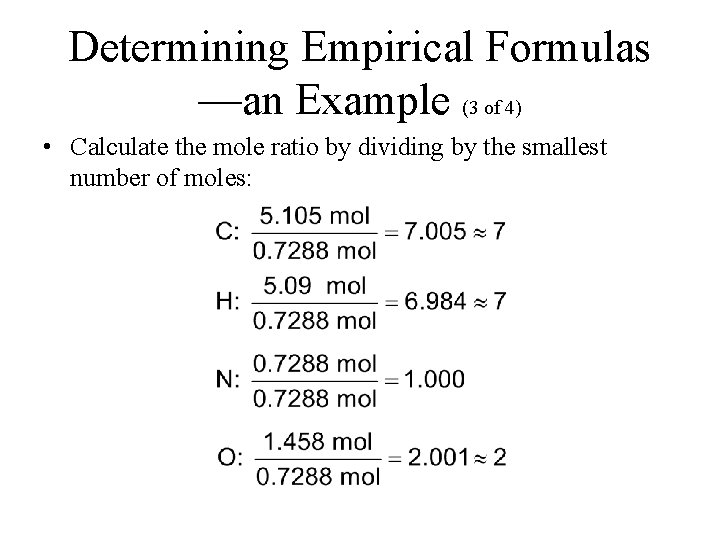
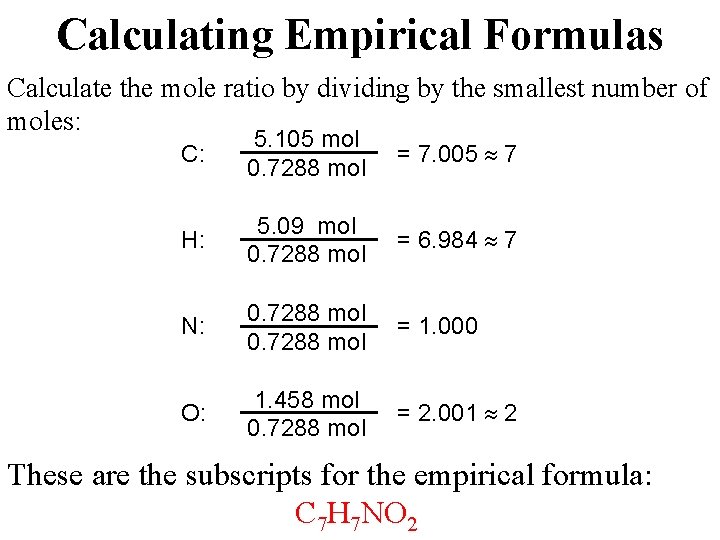
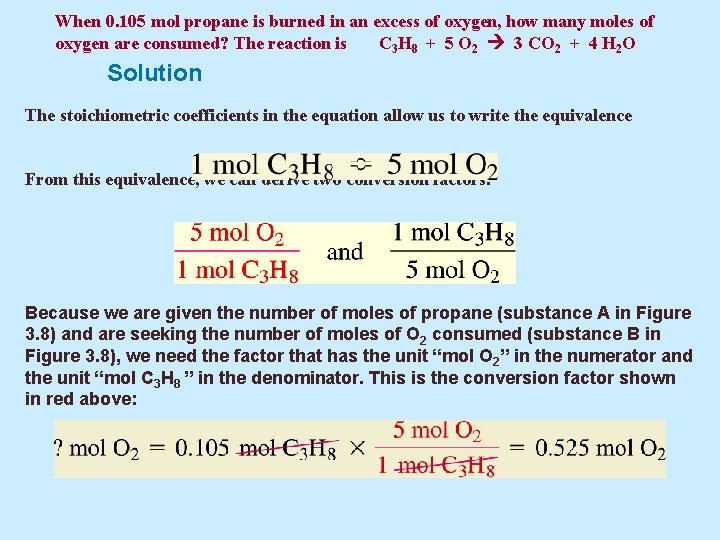
Each mole of the salt will contain one mole of Calcium and two moles of Chlorine. Atomic mass of calcium 40u. How many moles are 5 grams of calcium. 256 x 10 24 g b. 05 moles Submitted by. The atomic mass of CaCl2 is 401 2355 1111Amount ofCaCl2 mass of pure samplemolar mass 021111 000180molThere are 000180 moles of CaCl2 in a 02 gram pure sample.
2 moles Calcium Chloride to grams 221968 grams.
Grams mole molar mass. 2 moles Calcium Chloride to grams 221968 grams. How many moles of water are present. 1 mole is equal to 1 moles Calcium Chloride or 110984 grams. About Calcium chloride dihydrate. To be able to convert the moles of a substance to grams you will have to multiply the mole worth of the substance by its molar mass.
 Source: slideplayer.com
Source: slideplayer.com
Moles 2000 169873 1177 moles. Molarity is 20M so the number of moles is 0125 x 2 025 mol. For every 1000ml of water the weight should be 1000grams. Each mole of the salt will contain one mole of Calcium and two moles of Chlorine. Similarly what is the mass of one mole of calcium chloride.
 Source: slideplayer.com
Source: slideplayer.com
One mole of a substance contains 60231023 formula units. What number of moles are in 5 grams of calcium chloride. Density of calcium chloride dihydrate is equal to 1 850 kgm³. So given 25 moles of CaCl2 when dissolved in a suitable solvent will give you 25 moles of Clacium ions 1x2. Quick conversion chart of moles Calcium Chloride to grams.
 Source: youtube.com
Source: youtube.com
Answer 1 of 2. Atomic mass of calcium 40u. Molarity is 20M so the number of moles is 0125 x 2 025 mol. To be able to convert the moles of a substance to grams you will have to multiply the mole worth of the substance by its molar mass. One may also ask how many moles of chloride ions are there in 2 moles of calcium chloride.
 Source: slideplayer.com
Source: slideplayer.com
There are 90108 millimoles in 100 grams of Calcium chloride. Thus there are 00450 moles of. Similarly what is the mass of one mole of calcium chloride. So given 25 moles of CaCl2 when dissolved in a suitable solvent will give you 25 moles of Clacium ions 1x2. So given 25 moles of CaCl2 when dissolved in a suitable solvent will give you 25 moles of Clacium ions 1x2.
 Source: slidetodoc.com
Source: slidetodoc.com
The molecular formula for Calcium Chloride is CaCl2 ie. The SI base unit for amount of substance is the mole. 1molecule of Calcium chloride consists of 1 Ca2 ion and a pair of Cl- ions. How many moles in 100 grams of Calcium chloride. How many moles of CaC12 are in solution.
 Source: slidetodoc.com
Source: slidetodoc.com
To be able to convert the moles of a substance to grams you will have to multiply the mole worth of the substance by its molar mass. Molarity is 20M so the number of moles is 0125 x 2 025 mol. I ooog mol 110 b. 05 moles Submitted by. If a lab procedure asks for 425 moles of calcium chloride how many grams are needed.
 Source: pinterest.com
Source: pinterest.com
Do a quick conversion. 5 500 ml 1gramml 25grams. 1molecule of Calcium chloride consists of 1 Ca2 ion and a pair of Cl- ions. Moles Worksheet 800 kg of calcium chloride CaC12 is dissolved in I 000 kg of water. Also what is the mass of 53 moles of CaCl2.
 Source: slidetodoc.com
Source: slidetodoc.com
256 x 10 24 g b. To calculate the moles we use the equation. The molecular formula for Calcium Chloride is CaCl2 ie. The molecular formula for Calcium Chloride is CaCl2 ie. So given 25 moles of CaCl2 when dissolved in a suitable solvent will give you 25 moles of Clacium ions 1x2.
 Source: slideplayer.com
Source: slideplayer.com
Calcium chloride is made from 1 calcium and 2 chlorine so the molecular mass would be 140 2355 111 grammoles. The molecular formula for Calcium Chloride is CaCl2 ie. The molecular formula for Calcium Chloride is CaCl2 ie. Atomic mass of calcium 40u. To calculate the moles we use the equation.
 Source: slideplayer.com
Source: slideplayer.com
Density of calcium chloride dihydrate is equal to 1 850 kgm³. 2 moles Calcium Chloride to grams 221968 grams. 256 x 10 24 g b. Moles Worksheet 800 kg of calcium chloride CaC12 is dissolved in I 000 kg of water. Molar mass of NaCl is 58443 how many grams is 5 mole NaCl.
 Source: slidetodoc.com
Source: slidetodoc.com
3 moles Calcium Chloride to grams 332952 grams. The answer of How many moles are in 222 grams of Calcium chloride CaCl2. Now simply multiply this by the molar mass of calcium chloride which is. 1 mole is equal to 1 moles MgCl2 or 95211 grams. The SI base unit for amount of substance is the mole.
 Source: cz.pinterest.com
Source: cz.pinterest.com
Step by step solution by experts to help you in doubt clearance scoring excellent marks in exams. How many moles are 5 grams of calcium. For every 1000ml of water the weight should be 1000grams. 00383 g Solution 425 mol CaCl 2 110984 g CaCl 2 1 mol CaCl 2 471682 g à 472 g Answer. 256 x 10 24 g b.
 Source: slideplayer.com
Source: slideplayer.com
Now simply multiply this by the molar mass of calcium chloride which is. The molar mass of calcium is 40 gmole. Consequently how many moles of chloride ions are there in 2 moles of calcium chloride. How many moles in 100 grams of Calcium chloride. Also what is the mass of 53 moles of CaCl2.
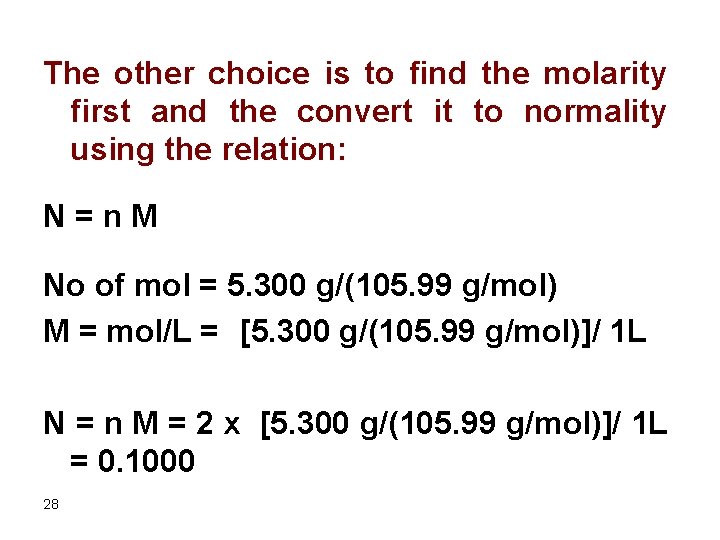 Source: slidetodoc.com
Source: slidetodoc.com
How many moles are 5 grams of calcium. How many moles in 100 grams of Calcium chloride. 05 moles Submitted by. For every 1000ml of water the weight should be 1000grams. Calcium chloride is made from 1 calcium and 2 chlorine so the molecular mass would be 140 2355 111 grammoles.
 Source: in.pinterest.com
Source: in.pinterest.com
Density of calcium chloride dihydrate is equal to 1 850 kgm³. Grams 58443 5 292215 g Molar mass of AgNO3 is 169873 2 kg AgNO3 is equal to how many moles. 1 mole is equal to 1 moles Calcium Chloride or 110984 grams. The mass in grams of a compound is equal to its molarity in moles multiply its molar mass. Furthermore how many moles of chloride ions are there in 2 moles of calcium chloride.
This site is an open community for users to do submittion their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site good, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title how many moles are in 5 grams of calcium chloride by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






