Your How many moles are in 28 grams of nitrogen images are ready. How many moles are in 28 grams of nitrogen are a topic that is being searched for and liked by netizens today. You can Find and Download the How many moles are in 28 grams of nitrogen files here. Find and Download all free images.
If you’re looking for how many moles are in 28 grams of nitrogen images information related to the how many moles are in 28 grams of nitrogen interest, you have pay a visit to the right site. Our site always provides you with suggestions for viewing the maximum quality video and image content, please kindly surf and find more informative video content and images that fit your interests.
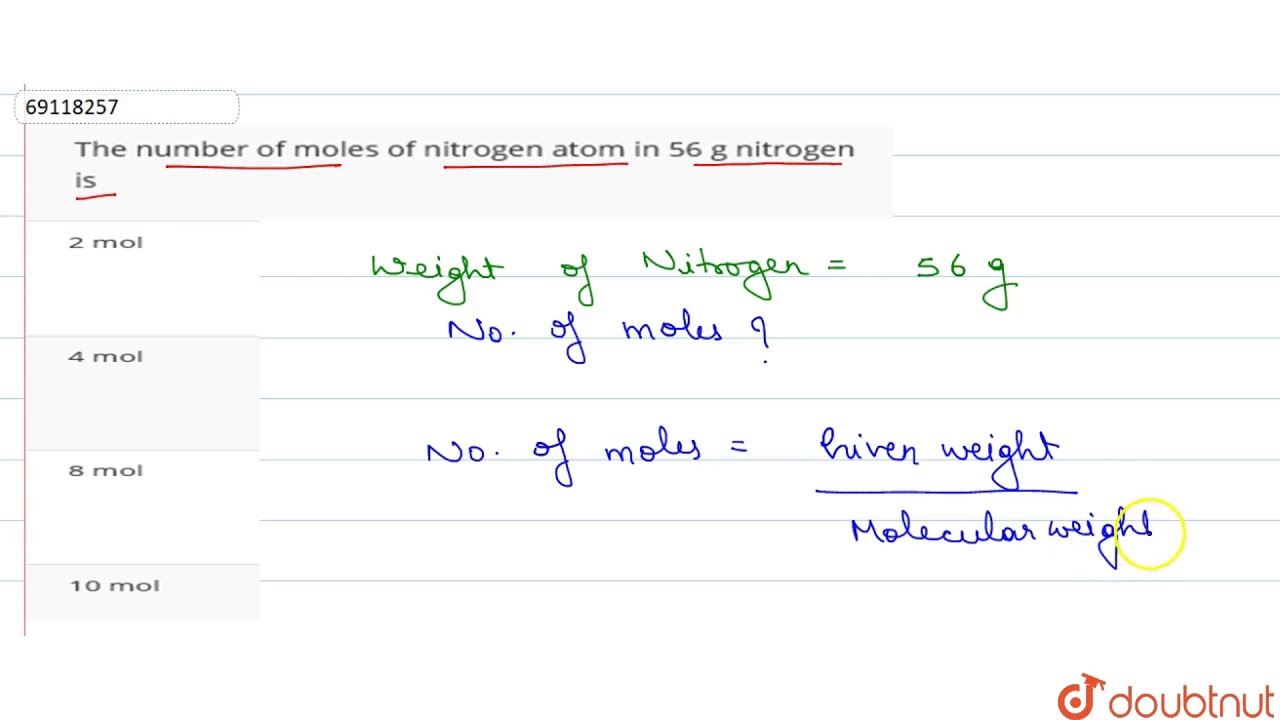
How Many Moles Are In 28 Grams Of Nitrogen. 1 C 1 x 1201 g 1201 g. Of moles Given mass molar mass. We assume you are converting between grams Nitrogen and mole. Moles N —– 2802 g 2802 x 1 140 2014 moles of N.
 Mass Moles And Molar Mass Read Pp 80 From slidetodoc.com
Mass Moles And Molar Mass Read Pp 80 From slidetodoc.com
So at STP or Standard Temperature and Pressure one mole of any ideal gas occupies exactly 227 L - this is known as the molar volume of a gas at STP. What would be the mass in grams of 450 liters or dm³of nitrogen gas at RTP. 1 mole of nitrogen has a mass of 2802 g while 3 moles of hydrogen have a mass of 606 g and 2 moles of ammonia have a mass of 3408 g. 1514 L N 2 x 1000 ml L x 0629 g ml 9523 g N 2 x 1 mol N 2 28 g 340. 4 moles Nitrogen to grams 560268 grams. 1 mole is equal to 1 moles Nitrogen Dioxide or 460055 grams.
We assume you are converting between grams Nitrogen Trifluoride and mole.
1mole of NH3 17 g Atomicity is defined as the total number of atoms that constitute a molecule. 5 moles Nitrogen to grams 700335 grams. Of moles 1428 05 moles. 2802 g N2 1 mol N2 1 mol H2 2 2 KClO3 2 KCl 3 O2 a How many grams of potassium chloride are produced if 25 grams of potassium chlorate decompose. Now use sodium azides molar amss to see how many grams would contain this many moles. You can view more details on each measurement unit.
 Source: youtube.com
Source: youtube.com
Molecular weight of Nitrogen Trifluoride or mol. Willchemistry Willchemistry Atomic mass N 140 uma 1 mole N —– 140 g. Add your answer and earn points. One mole of atomic nitrogen would have a mass of 14 grams. 1 mole of nitrogen contain number of atoms.
 Source: slidetodoc.com
Source: slidetodoc.com
The molecular formula for Nitrogen Dioxide is NO2. 1 moles Nitrogen to grams 140067 grams. 25 g KClO3 1 mol KClO3 2 mol KCl 7455 g KCl 15 g KCl 12255 g KClO3 2 mol KClO3 1 mol KCl b How many grams of oxygen are produced from 25 grams of potassium. Molar mass of nitrogen molecule N2 is 28g. How many grams of hydrogen are needed to fully react 6219 grams of nitrogen gas.
 Source: youtube.com
Source: youtube.com
What would be the mass in grams of 450 liters or dm³of nitrogen gas at RTP. How many litres of nitrogen tihydride are produced at STP if 8028 grams of hydrogen gas are reacted in an excess of nitrogen. So number of moles of nitrogen is-There is 5 moles of nitrogen in 140g nitrogen. 3 moles Nitrogen to grams 420201 grams. How many moles in 28 grams of CO2.
 Source: youtube.com
Source: youtube.com
You can view more details on each measurement unit. Do a quick conversion. Number of moles Volume24 dm³ number of molecules 6022 x 10²³ Hence mass n x Mr 1875 x 28525g. How many litres of nitrogen tihydride are produced at STP if 8028 grams of hydrogen gas are reacted in an excess of nitrogen. 2802 g N2 1 mol N2 1 mol H2 2 2 KClO3 2 KCl 3 O2 a How many grams of potassium chloride are produced if 25 grams of potassium chlorate decompose.
 Source: pinterest.com
Source: pinterest.com
Molar mass of nitrogen molecule N2 is 28g. 6400 gmol 28 g CO2 064 moles CO2. Willchemistry Willchemistry Atomic mass N 140 uma 1 mole N —– 140 g. 5 mole of nitrogen will contain-So atoms are present in 140g nitrogen. Rounded to two sig figs the number of sig figs you gave for the temperature and volume of the gas the answer will be.
 Source: brainly.in
Source: brainly.in
One mole of atomic nitrogen would have a mass of 14 grams. One mole of atomic nitrogen would have a mass of 14 grams. Now use sodium azides molar amss to see how many grams would contain this many moles. Number of moles of nitrogen is given by. How many atoms are in 0750 moles of zinc.
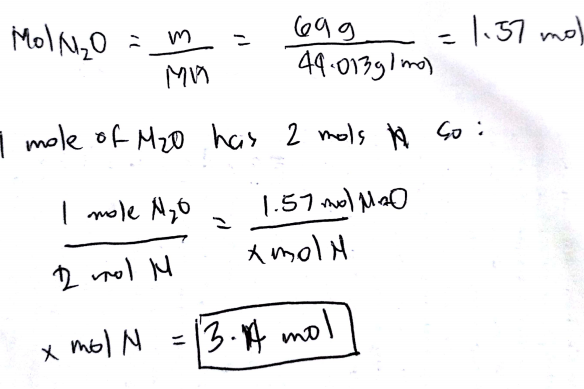 Source: clutchprep.com
Source: clutchprep.com
N2 3H2 - 2NH3. How many litres of nitrogen tihydride are produced at STP if 8028 grams of hydrogen gas are reacted in an excess of nitrogen. Number of moles Volume24 dm³ number of molecules 6022 x 10²³ Hence mass n x Mr 1875 x 28525g. The molecular formula for Nitrogen Trifluoride is NF3. Of moles 1428 05 moles.
 Source: youtube.com
Source: youtube.com
Willchemistry Willchemistry Atomic mass N 140 uma 1 mole N —– 140 g. The answer is 140067. Molar mass of nitrogen molecule N2 is 28g. 25 g KClO3 1 mol KClO3 2 mol KCl 7455 g KCl 15 g KCl 12255 g KClO3 2 mol KClO3 1 mol KCl b How many grams of oxygen are produced from 25 grams of potassium. The SI base unit for amount of substance is the mole.
 Source: pinterest.com
Source: pinterest.com
How many moles in 28 grams of CO2. The molecular formula for Nitrogen Trifluoride is NF3. Rounded to two sig figs the number of sig figs you gave for the temperature and volume of the gas the answer will be. 2 moles Nitrogen to grams 280134 grams. We assume you are converting between grams Nitrogen and mole.
 Source: pinterest.com
Source: pinterest.com
The weight of the 02 mol atoms is 16 02 which corresponds to 32 grams. The molecular formula for Nitrogen Dioxide is NO2. 1 mole of nitrogen contain number of atoms. 5 mole of nitrogen will contain-So atoms are present in 140g nitrogen. Divide the molar mass into the given mass to get the number of moles present.
 Source: youtube.com
Source: youtube.com
3408g 1mol of nitrogen has a mass of 2802g while 3mol of hydrogen has a mass of 606g and 2mol of ammonia has a mass of 3408g. Rounded to two sig figs the number of sig figs you gave for the temperature and volume of the gas the answer will be. The molecular formula for Nitrogen Dioxide is NO2. Now number of moles is a ratio of mass to molecular mass. 3408g 1mol of nitrogen has a mass of 2802g while 3mol of hydrogen has a mass of 606g and 2mol of ammonia has a mass of 3408g.
 Source: pinterest.com
Source: pinterest.com
The answer is 710019096. Molecular weight of Nitrogen or mol The molecular formula for Nitrogen is N. 1514 L N 2 x 1000 ml L x 0629 g ml 9523 g N 2 x 1 mol N 2 28 g 340. So at STP or Standard Temperature and Pressure one mole of any ideal gas occupies exactly 227 L - this is known as the molar volume of a gas at STP. 1 mole is equal to 1 moles Nitrogen Dioxide or 460055 grams.
 Source: toppr.com
Source: toppr.com
How many litres of nitrogen tihydride are produced at STP if 8028 grams of hydrogen gas are reacted in an excess of nitrogen. You can view more details on each measurement unit. 5430 x 10 ²³ Therefore 100 g of N2 Contains 4310²³ atoms Calculating Number of atoms in NH3. 1 mole of nitrogen has a mass of 2802 g while 3 moles of hydrogen have a mass of 606 g and 2 moles of ammonia have a mass of 3408 g. There is 1 mole of O2 in 32 grams of O2 c.
 Source: slideplayer.com
Source: slideplayer.com
6 moles Nitrogen to grams 840402 grams. 4 moles Nitrogen to grams 560268 grams. There is 1 mole of O2 in 32 grams of O2 c. How many grams are in 2 moles of NH3. How many grams of hydrogen are needed to fully react 6219 grams of nitrogen gas.
 Source: youtube.com
Source: youtube.com
1 mole of nitrogen has a mass of 2802 g while 3 moles of hydrogen have a mass of 606 g and 2 moles of ammonia have a mass of 3408 g. Molecular weight of Nitrogen Trifluoride or mol. How many moles are in nitrogen gas. Divide the molar mass into the given mass to get the number of moles present. Number of moles of nitrogen is given by.
This site is an open community for users to submit their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site helpful, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title how many moles are in 28 grams of nitrogen by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






