Your How many moles are in 28 grams of co2 images are available. How many moles are in 28 grams of co2 are a topic that is being searched for and liked by netizens now. You can Download the How many moles are in 28 grams of co2 files here. Download all royalty-free photos and vectors.
If you’re looking for how many moles are in 28 grams of co2 pictures information related to the how many moles are in 28 grams of co2 topic, you have visit the ideal site. Our site frequently provides you with suggestions for refferencing the maximum quality video and picture content, please kindly search and locate more informative video articles and graphics that match your interests.
How Many Moles Are In 28 Grams Of Co2. Find the grams in 126 x 10-4 mol of HC2H3O2. 2 130 moles O. CO2 has a molecular weight of one carbon 12 plus two oxygen2x16 or 44. Gram-formula-mass of CO 2 1 C 1 x 1201 g 1201 g 2 O 2 x 1600 g 3200 g 6400 gmol 28 g CO 2.
 Calculate The Moles Of Carbon In G Of Pencil Lead Ppt Download From slideplayer.com
Calculate The Moles Of Carbon In G Of Pencil Lead Ppt Download From slideplayer.com
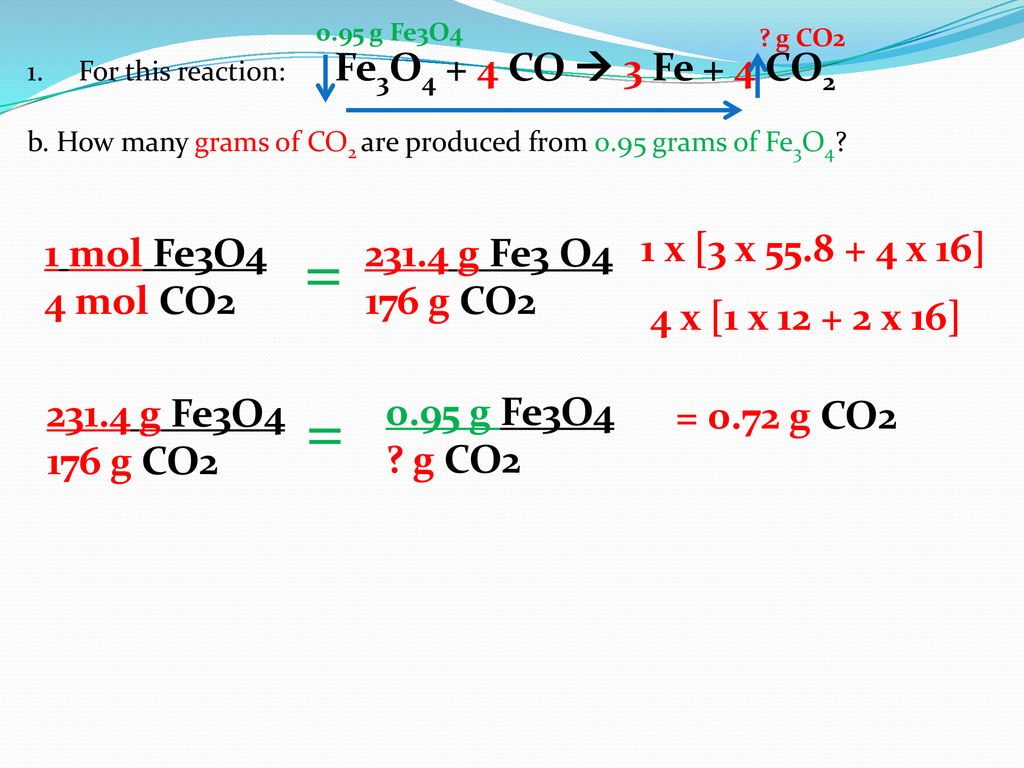
How many moles of C. How many moles Co2 in 1 grams. How many moles of propane must be burned in order to produce 037 g C02. What is the mass of 5 moles of Fe2O3. How many moles in 28 grams of CO2. How many grams of O 2 must react with excess.
What is the mass of 5 moles of Fe2O3.
We assume you are converting between grams CO2 and mole. A gram-mole is one gram times the molecular weight soa gram mole of CO2 is 44 grams. Click here to get an answer to your question how many moles are in 28 grams of co2 taurihill16 taurihill16 01092018 Chemistry High School answered How many moles are in 28 grams of co2 2 See answers Advertisement. How many moles in 28 grams of CO2. 1 C 1 x 1201 g 1201 g. At what temperature in o C does 0398 moles of gas occupy a volume of 111 Liters at a pressure of 0980 atm.
 Source: slideplayer.com
Source: slideplayer.com
Find the number of moles of argon in 452 g of argon. What is the mass of 5 moles of Fe2O3. A gram-mole is one gram times the molecular weight soa gram mole of CO2 is 44 grams. The answer is 00084841820909097. What is the mass of 5 moles of Fe2O3.

The answer is 00084841820909097. 2 O 2 x 1600 g 3200 g. 00079 grams or 79 x 10-3 grams. The numeric value of this constant is 6022 10 23. Click here to get an answer to your question how many moles are in 28 grams of co2 taurihill16 taurihill16 01092018 Chemistry High School answered How many moles are in 28 grams of co2 2 See answers Advertisement.
 Source: slideplayer.com
Source: slideplayer.com
You can view more details on each measurement unit. How many moles in 28 grams of CO2. Find the mass in 26 mol of lithium bromide. Find the molecular weight of Carbon monoxide. Gram-formula-mass of CO 2 1 C 1 x 1201 g 1201 g 2 O 2 x 1600 g 3200 g 6400 gmol 28 g CO 2.
 Source: slideplayer.com
Source: slideplayer.com
The SI base unit for amount of substance is the mole. Determine the volume in liters occupied by 0030 moles of a gas at STP. Find the number of moles of argon in 452 g of argon. 3 O 3 x 160 g 480 g. Molecular weight of CO2 or grams This compound is also known as Carbon Dioxide.
 Source: slideplayer.com
Source: slideplayer.com
3 O 3 x 160 g 480 g. The SI base unit for amount of substance is the mole. 15 6022 10 23. How many moles of propane must be burned in order to produce 037 g C02. 2 O 2 x 1600 g 3200 g.

G 4 002 037564 x 3 662 4409 CC How many grams of propane is this. - Firstly we will write the given mass as. Molecular weight of Co2 or grams The SI base unit for amount of substance is the mole. What volume of Cl 2 gas is occupied by 111 grams at 250 o C and 740 mm Hg. 1 C 1 x 1201 g 1201 g.
 Source: slideplayer.com
Source: slideplayer.com
Find the molecular weight of Carbon monoxide. Molecular weight of CO2 or grams This compound is also known as Carbon Dioxide. G N 2 947 g H 2 x 1 mol H 2 x 1 mol N 2 x 2801g N 2 2016 g H 2 3 mol H 2 1 molN2 439 g N 2 Stoichiometry CH 4 2 O 2 CO 2 2 H 2O Answer the following questions. 1mol H2O 180g X 2mol C8H18 18mol H2O 123 x 10-2 mol C8H18 Answer _____ c. How many molecules are in 0400 moles of N2O5.
 Source: slideplayer.com
Source: slideplayer.com
2 Fe 2 x 556 g 1112 g. 00079 grams or 79 x 10-3 grams. How many grams of O 2 must react with excess. 2 O 2 x 1600 g 3200 g. How many moles in 28 grams of CO2.
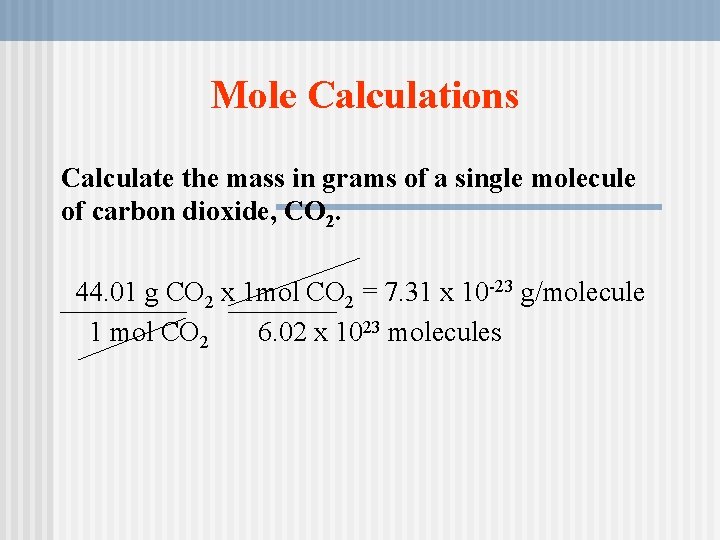 Source: slidetodoc.com
Source: slidetodoc.com
How many moles of C. 2 130 moles O. 15 6022 10 23. G N 2 947 g H 2 x 1 mol H 2 x 1 mol N 2 x 2801g N 2 2016 g H 2 3 mol H 2 1 molN2 439 g N 2 Stoichiometry CH 4 2 O 2 CO 2 2 H 2O Answer the following questions. Must be used to produce 199 grams of water.
 Source: youtube.com
Source: youtube.com
How many moles Co2 in 1 grams. The SI base unit for amount of substance is the mole. Since we need to find for 3 moles of Carbon monoxide 3 x 2801 8403 grams. You can view more details on each measurement unit. 2802 g N2 1 mol N2 1 mol H2.
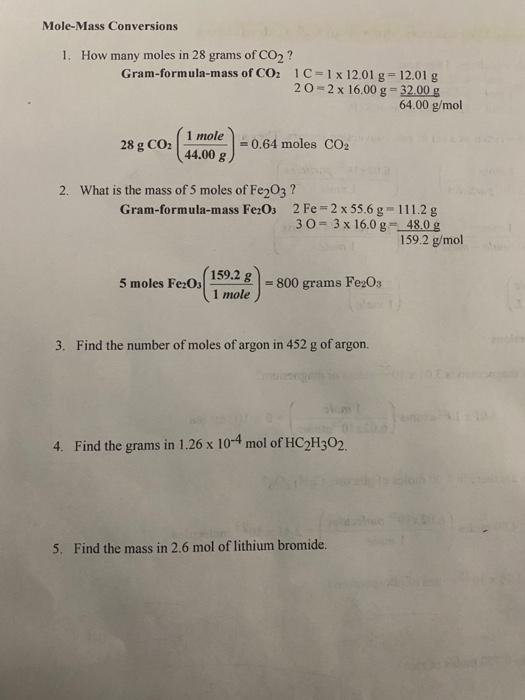
We assume you are converting between grams CO2 and mole. Molecular weight of CO2 or grams This compound is also known as Carbon Dioxide. What is the mass of 5 moles of Fe2O3. What is the mass of 5 moles of Fe2O3. How many grams of H 2O will form when 109 moles of CH 4 react with excess O 2.
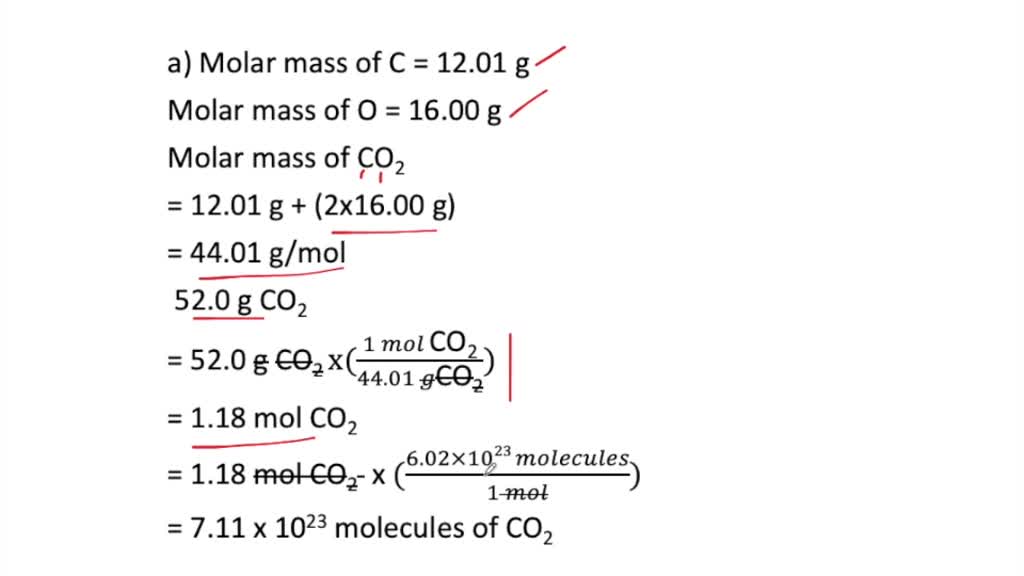 Source: numerade.com
Source: numerade.com
Find the grams in 126 x 10-4 mol of HC2H3O2. You can view more details on each measurement unit. 1mol H2O 180g X 2mol C8H18 18mol H2O 123 x 10-2 mol C8H18 Answer _____ c. How many moles of hydrogen are produced from the reaction of three moles of zinc with an excess of HCl. 2 130 moles O.
 Source: youtube.com
Source: youtube.com
1 grams CO2 is equal to 0022722366761722 mole. What volume of Cl 2 gas is occupied by 111 grams at 250 o C and 740 mm Hg. Since we need to find for 3 moles of Carbon monoxide 3 x 2801 8403 grams. You can view more details on each measurement unit. Find the molecular weight of Carbon monoxide.
 Source: slideplayer.com
Source: slideplayer.com
We assume you are converting between moles Co2 and gram. 6400 gmol 28 g CO2 064 moles CO2. 2 O 2 x 1600 g 3200 g. The answer is 440095. Determine the volume in liters occupied by 0030 moles of a gas at STP.
 Source: slideplayer.com
Source: slideplayer.com
CO2 has a molecular weight of one carbon 12 plus two oxygen2x16 or 44. 2 O 2 x 1600 g 3200 g. Convert 3 moles of carbon monoxide to grams. How many moles of O 2 are required to react with 172 moles of CH 4. How many moles of hydrogen are produced from the reaction of three moles of zinc with an excess of HCl.
This site is an open community for users to submit their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site beneficial, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title how many moles are in 28 grams of co2 by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






