Your How many moles are in 12 grams of aluminum images are available in this site. How many moles are in 12 grams of aluminum are a topic that is being searched for and liked by netizens today. You can Get the How many moles are in 12 grams of aluminum files here. Find and Download all royalty-free vectors.
If you’re searching for how many moles are in 12 grams of aluminum pictures information related to the how many moles are in 12 grams of aluminum interest, you have visit the right blog. Our website frequently provides you with suggestions for viewing the maximum quality video and picture content, please kindly search and locate more informative video articles and graphics that fit your interests.
How Many Moles Are In 12 Grams Of Aluminum. Notice that the value 1201 grams of natural carbon is the same as the atomic mass value 1201 amu. It also tells us that 2698 grams of aluminum contains exactly 6022 x 10 23 atoms of aluminum. It means 1 mole of aluminium contains 27g of aluminium. 120 x 1024 atoms Li.
 Chemical Quantities The Mole Ppt Download From slideplayer.com
Chemical Quantities The Mole Ppt Download From slideplayer.com
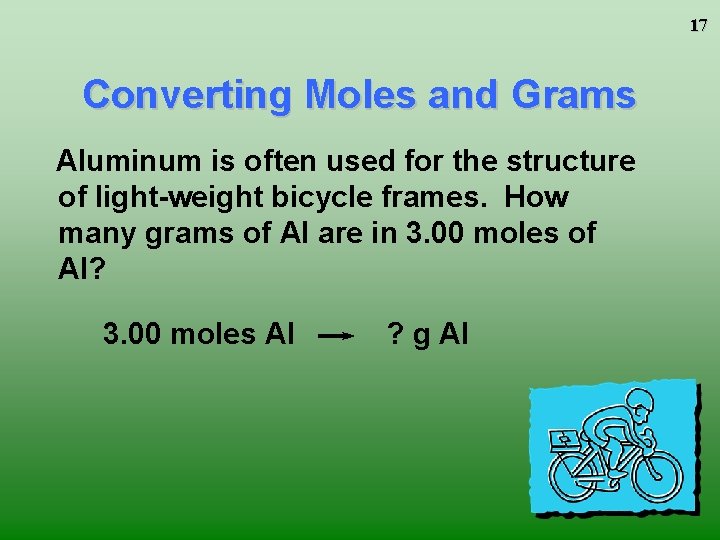
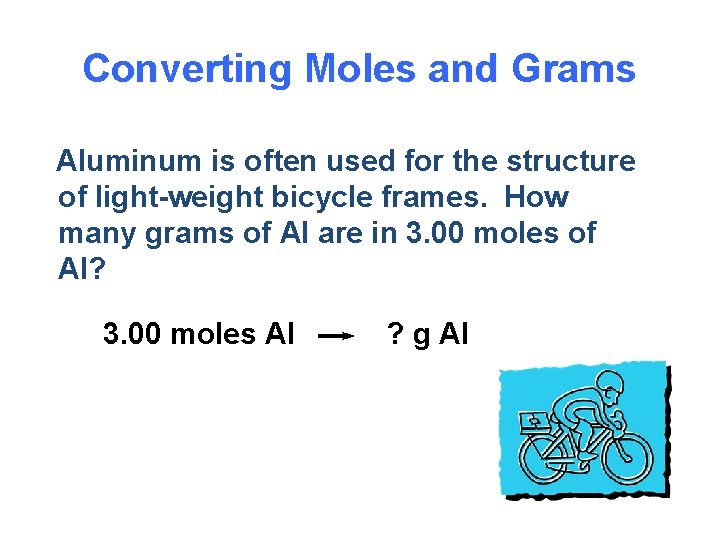
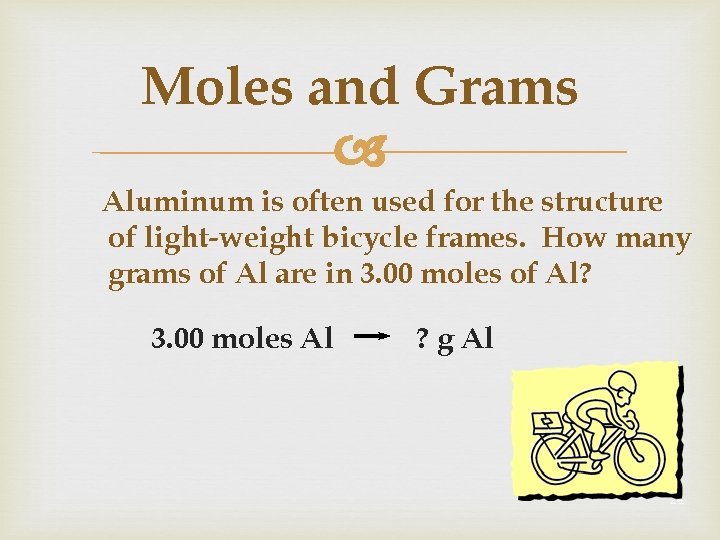
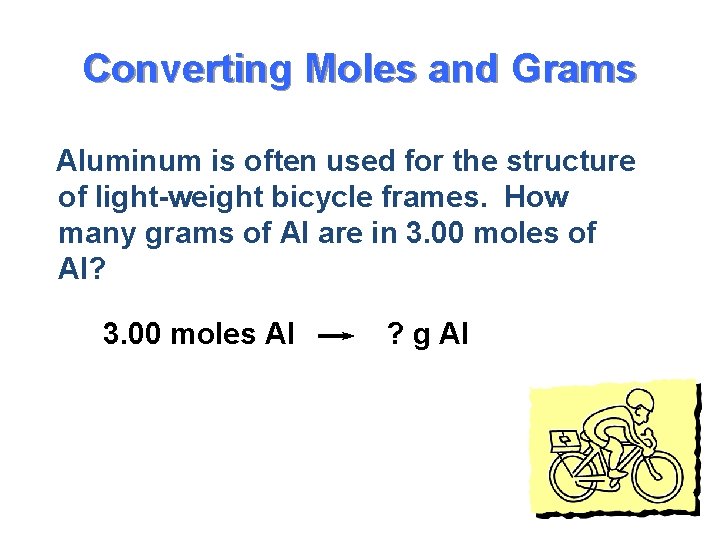
One mole of any substance is equal to the value of 6023 x 1023 Avagadro number. 1 mole of aluminium 27g 60231023 noof atoms 27g of aluminium contains 60231023 noof atoms Therefore10g602310231027 2231023 noof. How many moles are in 12g of aluminum. When aluminum is burned in excess oxygen aluminum oxide is produced. Molecular weight of aluminum or mol The molecular formula for aluminum is Al. The molecular formula for Aluminum is Al.
C 5H 12g 8 O 2g 5 CO 2 g 6 H 2Og 100.
Taffy927x2 and 4 more users found this answer helpful. Mole constant atoms of any element. We assume you are converting between grams aluminum and mole. We assume you are converting between grams Aluminum and mole. Secondly how many atoms are present in 9g of Aluminium. For example if we want to total the molar mass of Aluminum Sulfate Al 2 SO 4 3 we need to determine the number and mass of each element in the compound.
 Source: pinterest.com
Source: pinterest.com
How many grams of water are needed to react with 1000 moles of Al4C3. 044 mol Al. For example if we want to total the molar mass of Aluminum Sulfate Al 2 SO 4 3 we need to determine the number and mass of each element in the compound. 13 How many grams are in 33 moles of potassium sulfide K S. Notice that the value 1201 grams of natural carbon is the same as the atomic mass value 1201 amu.
 Source: slidetodoc.com
Source: slidetodoc.com
The SI base unit for amount of substance is the mole. Density of aluminum is equal to 2 699 kgm³. How many grams of water are needed to react with 1000 moles of Al4C3. 11 How many grams are in 002 moles of beryllium iodide Bels. 412 grams aluminum sulfate is equivalent to 0012 moles for the anhydrous salt.
 Source: slideplayer.com
Source: slideplayer.com
The SI base unit for amount of substance is the mole. Convert moles to grams and grams to moles. The answer is 26981538. 0185 How many grams of aluminum oxide are produced according to the reaction below given that you. 12 How many moles are in 68 grams of copper IT hydroxide CuOH2.
 Source: pinterest.com
Source: pinterest.com
Is defined as th the mass of a neutral carbon-12 atom. Furthermore how many atoms are in a gram of aluminum. The balanced equation is. 1000mol Al4C3 X. We assume you are converting between grams Aluminum and mole.
 Source: toppr.com
Source: toppr.com
One mole of any substance is equal to the value of 6023 x 1023 Avagadro number. Taffy927x2 and 4 more users found this answer helpful. Density of aluminum is equal to 2 699 kgm³. G C 5H 12 139 mol C 5H 12 Step 1. It means 1 mole of aluminium contains 27g of aluminium.
 Source: slidetodoc.com
Source: slidetodoc.com
The answer is 26981538. Therefore 241102 atomsmol of Aluminum are present in 4 mol of Aluminum. 14 How many moles are in 12 x 10 grams of ammonia NH3. That means that 12 g of Almath_2mathOmath_3math contain 12 g 10196 g molmath-1math 0118 moles of. Note that rounding errors may occur so always check the results.
 Source: pinterest.com
Source: pinterest.com
How many moles of Al4C3 were reacted when 355 x 10 35 formulas units of aluminum hydroxide were produced. One mole is equal to the Avogadros number and each element has a different molar mass depending on the weight. 1000mol Al4C3 X. How many atoms are in 4 moles of aluminum. How many atoms are in 25 moles.
 Source: slideplayer.com
Source: slideplayer.com
For example if we want to total the molar mass of Aluminum Sulfate Al 2 SO 4 3 we need to determine the number and mass of each element in the compound. How many moles of aluminum oxide are produced according to the reaction below given that you start with 100 grams of Al and 190 grams of O2. Use this page to learn. Notice that the value 1201 grams of natural carbon is the same as the atomic mass value 1201 amu. How many atoms are in 55 grams of iron.

Furthermore how many atoms are in a gram of aluminum. 1 mole of aluminium 27g 60231023 noof atoms 27g of aluminium contains 60231023 noof atoms Therefore10g602310231027 2231023 noof. 12 How many moles are in 68 grams of copper IT hydroxide CuOH2. Mole constant atoms of any element. 13 How many grams are in 33 moles of potassium sulfide K S.
 Source: slidetodoc.com
Source: slidetodoc.com
We assume you are converting between grams Aluminum and mole. Mole is the unit for the number of atoms or molecules of a material. We assume you are converting between grams aluminum and mole. The answer is 26981538. The SI base unit for amount of substance is the mole.
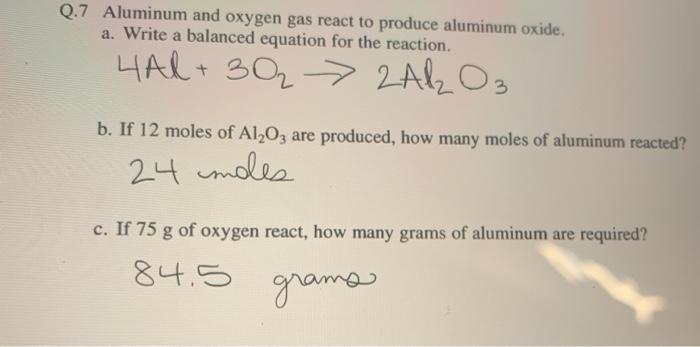
The answer is choice a. 11 How many grams are in 002 moles of beryllium iodide Bels. Secondly how many atoms are present in 9g of Aluminium. Mole constant atoms of any element. The molecular formula for Aluminum is Al.
 Source: slidetodoc.com
Source: slidetodoc.com
Taffy927x2 and 4 more users found this answer helpful. How many grams of ironIII chloride are produced when 153 g of iron react with excess chlorine gas. This is equal to the mass of. Molecular weight of aluminum or mol The molecular formula for aluminum is Al. 412 grams aluminum sulfate is equivalent to 0012 moles for the anhydrous salt.
 Source: slideplayer.com
Source: slideplayer.com
Molecular weight of aluminum or mol The molecular formula for aluminum is Al. It also tells us that 2698 grams of aluminum contains exactly 6022 x 10 23 atoms of aluminum. Note that rounding errors may occur so always check the results. 0185 How many grams of aluminum oxide are produced according to the reaction below given that you. When aluminum is burned in excess oxygen aluminum oxide is produced.
 Source: slidetodoc.com
Source: slidetodoc.com
The balanced equation is. So here is the math using the atomic masses given in the periodic table. Use this page to learn. 59 x 1023 atoms Fe. How many moles of Al4C3 were reacted when 355 x 10 35 formulas units of aluminum hydroxide were produced.
 Source: youtube.com
Source: youtube.com
Notice that the value 1201 grams of natural carbon is the same as the atomic mass value 1201 amu. Furthermore how many atoms are in a gram of aluminum. The SI base unit for amount of substance is the mole. 13 How many grams are in 33 moles of potassium sulfide K S. How many grams of ironIII chloride are produced when 153 g of iron react with excess chlorine gas.
This site is an open community for users to submit their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site beneficial, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title how many moles are in 12 grams of aluminum by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.