Your How many moles are in 100 grams of carbon images are available in this site. How many moles are in 100 grams of carbon are a topic that is being searched for and liked by netizens now. You can Find and Download the How many moles are in 100 grams of carbon files here. Find and Download all royalty-free photos and vectors.
If you’re searching for how many moles are in 100 grams of carbon pictures information connected with to the how many moles are in 100 grams of carbon topic, you have pay a visit to the ideal site. Our website frequently gives you suggestions for viewing the maximum quality video and image content, please kindly hunt and find more informative video content and graphics that fit your interests.
How Many Moles Are In 100 Grams Of Carbon. You can view more details on each measurement unit. Calcium carbonate decomposes at high temperature forming calcium oxide and carbon dioxide according to the following reaction. The molar mass of a substance depends not only on its molecular formula but also on the distribution of isotopes of each chemical element present in it. In water solution the concentration C in parts-per million ppm is equal to the concentration C in milligrams per kilogram mgkg and equal to 1000000 times the molar concentration molarity c in moles per liter molL times the solute molar mass in grams per mole gmol divided by the water solution density at temperature of 20ºC 998.
 David Chalk Teacherchalky1 Twitter Chemistry Lessons Chemistry Classroom Gcse Science From pinterest.com
David Chalk Teacherchalky1 Twitter Chemistry Lessons Chemistry Classroom Gcse Science From pinterest.com
10 grams of glucose C 6H 12O 6 react in a combustion reaction. 1 mole is equal to 1 moles In or 114818 grams. We assume you are converting between grams Carbon and mole. Calcium carbonate decomposes at high temperature forming calcium oxide and carbon dioxide according to the following reaction. We assume you are converting between moles Carbon Dioxide and gram. According to the Law of Conservation of Matter how many atoms will there be in the product of this reaction1 point A.
You can view more details on each measurement unit.
How many moles is 78 x 1025 molecules of phosphorus pentachloride. I want to correct it. 1 mole is equal to 1 moles In or 114818 grams. You can view more details on each measurement unit. If we start with 250 moles of butane how many moles of water will form. How many grams of water are needed to react with 1000 moles of Al4C3.
 Source: youtube.com
Source: youtube.com
Calculate the molar mass of the solute. When we do stoichiometry we always want to speak about chemicals in terms of how many. How many moles is 78 x 1025 molecules of phosphorus pentachloride. Therefore if you have 100 grams of methane it means that you have 10016625 moles of methane. 10 grams of glucose C 6H 12O 6 react in a combustion reaction.
 Source: pinterest.com
Source: pinterest.com
The formula shows the number of moles of each element. When 300 moles of hydrogen molecules and 150 moles of oxygen molecules react they form 300 moles of water according to the reaction below. For example 100 g of water is about 5551 mol of water. 1 mole is equal to 1 moles In or 114818 grams. Molecular weight of In or grams The SI base unit for amount of substance is the mole.

5g carbon 0417 mols of carbon 12 gmol How many moles of oxygen are in 12. The vapor pressure of carbon tetrachloride CCl 4 at 500 C is 0437 atm. 0411 atm 0437 atm x. Moles Units and Moles Units and Conversion FactorsConversion Factors. The molar mass is the sum of the masses of all the atoms in one mole of the compound.
 Source: pinterest.com
Source: pinterest.com
How many mols of are in 5g of carbon. How many moles of Al4C3 were reacted when 355 x 10 35 formulas units of aluminum hydroxide were produced. 10 grams of glucose C 6H 12O 6 react in a combustion reaction. How many grams is a mol. We assume you are converting between moles Carbon Dioxide and gram.
 Source: slidetodoc.com
Source: slidetodoc.com
CaCO 3 s CaO s CO 2 g If 100 kg of CaCO 3 is decomposed by this reaction how many kilograms. The vapor pressure of carbon tetrachloride CCl 4 at 500 C is 0437 atm. For example one atom of carbon has a mass of 12011 amu one mole of carbon has a mass of 12011 grams. Therefore if you have 100 grams of methane it means that you have 10016625 moles of methane. When you burn 625 moles of methane you produce 625 moles of carbondioxide.
 Source: pinterest.com
Source: pinterest.com
How many moles Carbon Dioxide in 1 grams. NaHCO3s atomic mass being 8401 grams made the conversion 1 mol equal to 8401g. 355 x 1035 formula units AlOH3 X. First we had to find the molar mass of baking soda sodium hydrogen carbonate NaHCO3. Molecular weight of Carbon Dioxide or grams The molecular formula for Carbon Dioxide is CO2.
 Source: slidetodoc.com
Source: slidetodoc.com
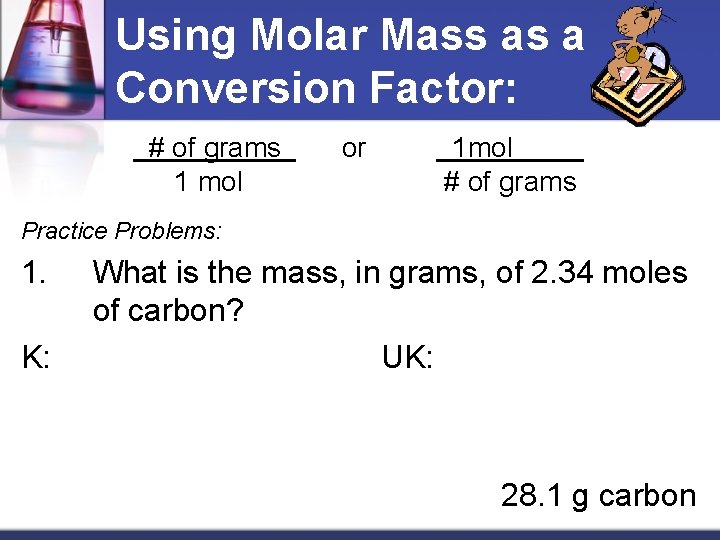
Gap the mass of every component by the molar mass and increase the outcome by 100. Molecular weight of In or grams The SI base unit for amount of substance is the mole. True False Question 2MultipleChoice Score. 1 grams Carbon is equal to 0083259093974539 mole. The formula shows the number of moles of each element.

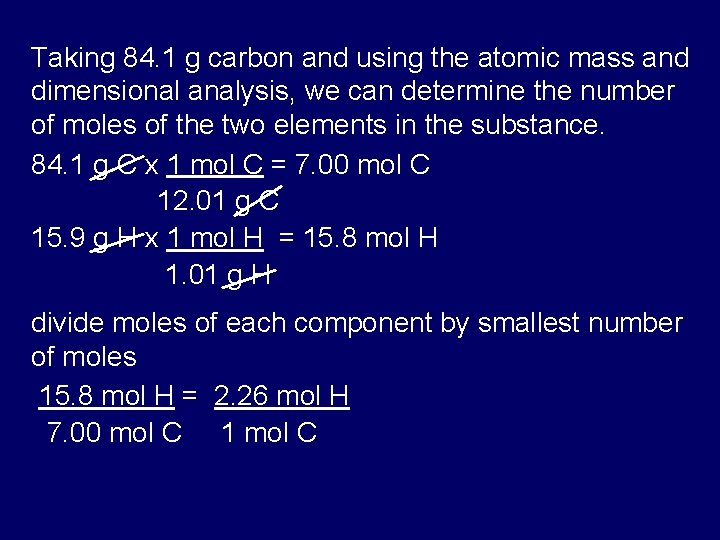
To do this we had to use the conversin factor of 1 mol the atomic mass of the molecule. Mass percent is also known as percent by weight or ww. This is known as the molar mass M and has the units g mol-1 gmol grams per mole of substance The relationship between molar mass mass and moles can be expressed as a mathematical equation as shown below. Calcium carbonate minerals are the building blocks for the skeletons and. 10 grams of glucose C 6H 12O 6 react in a combustion reaction.
 Source: slideplayer.com
Source: slideplayer.com
The number of grams in a mole differs from substance to substance just like a dozen eggs has a different weight than a dozen elephants a mole of oxygen has a different weight than a mole of hydrogen even though in each case there are 602 x 10 23 atoms. I want to correct it. How many grams of water are needed to react with 1000 moles of Al4C3. Mass percent is also known as percent by weight or ww. If we start with 250 moles of butane how many moles of water will form.
 Source: slideplayer.com
Source: slideplayer.com
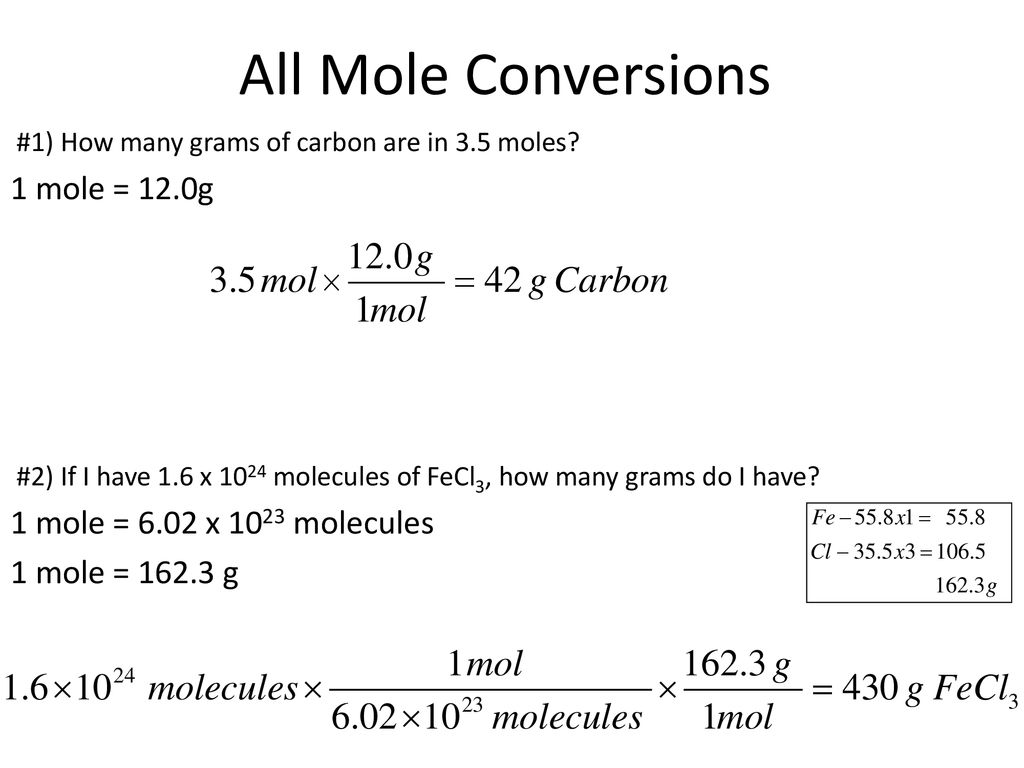
The sum of all the mass percentages should add up to 100. How many moles is 78 x 1025 molecules of phosphorus pentachloride. 10 grams of glucose C 6H 12O 6 react in a combustion reaction. Watch for rounding errors in the last significant figure to make sure all the percentages add up. When we do stoichiometry we always want to speak about chemicals in terms of how many.

The SI base unit for amount of substance is the mole. When you burn 625 moles of methane you produce 625 moles of carbondioxide. N2g 3 H 2g 2 NH 3g How many grams of NH 3 would. 5g carbon 0417 mols of carbon 12 gmol How many moles of oxygen are in 12. How many grams of water are needed to react with 1000 moles of Al4C3.

The molar mass is the sum of the masses of all the atoms in one mole of the compound. When you burn 625 moles of methane you produce 625 moles of carbondioxide. How many moles Carbon Dioxide in 1 grams. How many grams of each product are produced. Use the molar mass to convert from moles to grams The number of grams of a substance per mole Mass g Compound A Moles Compound A Moles Compound B Mass g Compound B Molar mass Molar mass Molar X ratio Moles and Chemical Reactions Chapter 4 Stoichiometry For the following reaction.
 Source: pinterest.com
Source: pinterest.com
The formula shows the number of moles of each element. Calculate the molar mass of the solute. The number of moles of a substance in a sample is obtained by dividing the mass of the sample by the molar mass of the compound. According to the Law of Conservation of Matter how many atoms will there be in the product of this reaction1 point A. The mass of 0100 mole of neon is 2018 grams.
 Source: youtube.com
Source: youtube.com
How many grams of water were formed. How many grams Carbon in 1 mol. When you burn 625 moles of methane you produce 625 moles of carbondioxide. People can find the molar mass or atomic mass of elements on the periodic table and the molar mass of a compound is the total molar mass of all. 12mol H2O 1mol Al4C3 X 1801g H2O 1mol H2O 2160 x 104g H2O.
 Source: slidetodoc.com
Source: slidetodoc.com
The answer is 0022722366761722. Use the molar mass to convert from moles to grams The number of grams of a substance per mole Mass g Compound A Moles Compound A Moles Compound B Mass g Compound B Molar mass Molar mass Molar X ratio Moles and Chemical Reactions Chapter 4 Stoichiometry For the following reaction. 1 grams Carbon is equal to 0083259093974539 mole. How many grams of NaCl are required to make 500mL of a 20M solution. We had to convert 05 moles of baking soda to grams.
This site is an open community for users to do submittion their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site beneficial, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title how many moles are in 100 grams of carbon by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.