Your How many moles are in 1 gram of copper images are available in this site. How many moles are in 1 gram of copper are a topic that is being searched for and liked by netizens today. You can Download the How many moles are in 1 gram of copper files here. Find and Download all free photos.
If you’re looking for how many moles are in 1 gram of copper pictures information related to the how many moles are in 1 gram of copper topic, you have come to the ideal blog. Our website frequently provides you with hints for viewing the maximum quality video and picture content, please kindly surf and find more informative video content and images that fit your interests.
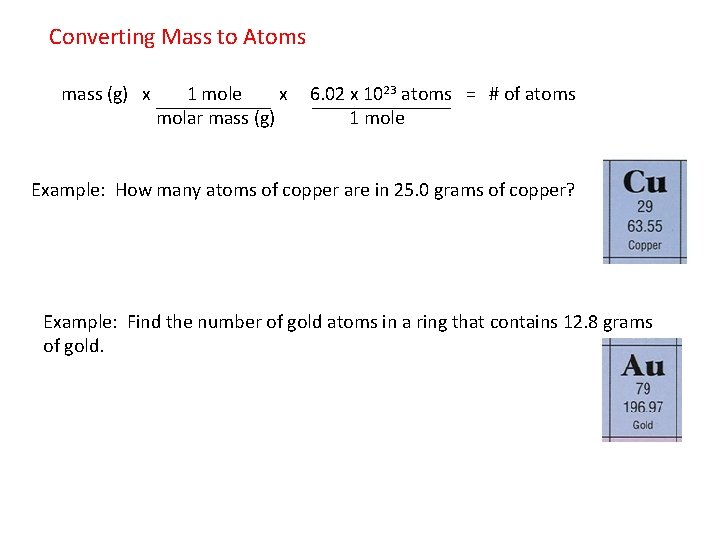
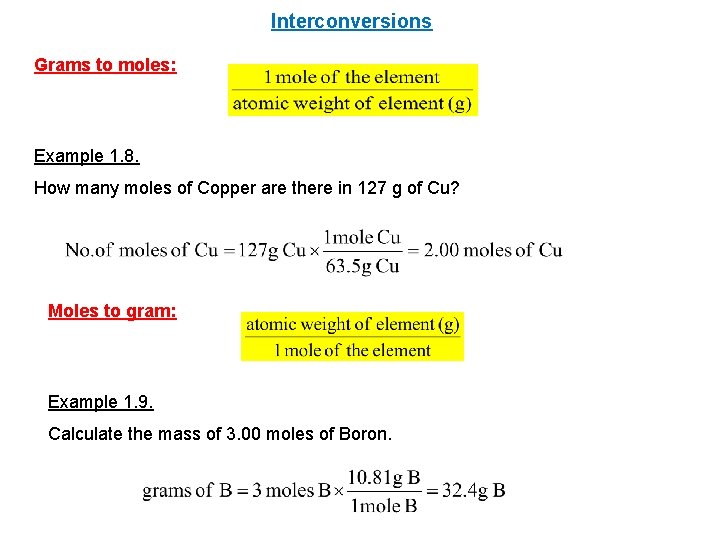
How Many Moles Are In 1 Gram Of Copper. How many GRAMS of copper II hydroxide are present in 223 moles of this compound. 1 10 -6. That is NA 60221023 Now apply UNITARY METHOD therefore mass of 1305 gram of Cu 60221023 1305 635 122 1023 atoms. The relation between molecular formula mass and molar mass Page 4 4 To obtain one mole of copper atoms 602 x 1023 atoms weigh out 6355 g copper.
 Chemical Calculations Formula Masses Moles And Chemical Equations From slidetodoc.com
Chemical Calculations Formula Masses Moles And Chemical Equations From slidetodoc.com
The relation between molecular formula mass and molar mass Page 4 4 To obtain one mole of copper atoms 602 x 1023 atoms weigh out 6355 g copper. Answer 1 of 4. The SI base unit for amount of substance is the mole. Density of copper is equal to 8 940 kgm³. That implies one gram copper contains 6023102363559481021 atoms approximately. The answer is 0015736631731344.
Note that the question gave the mass as 249.
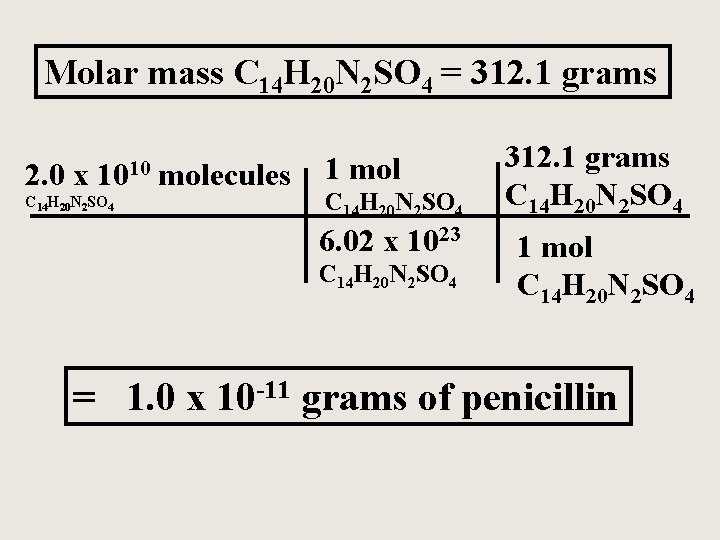
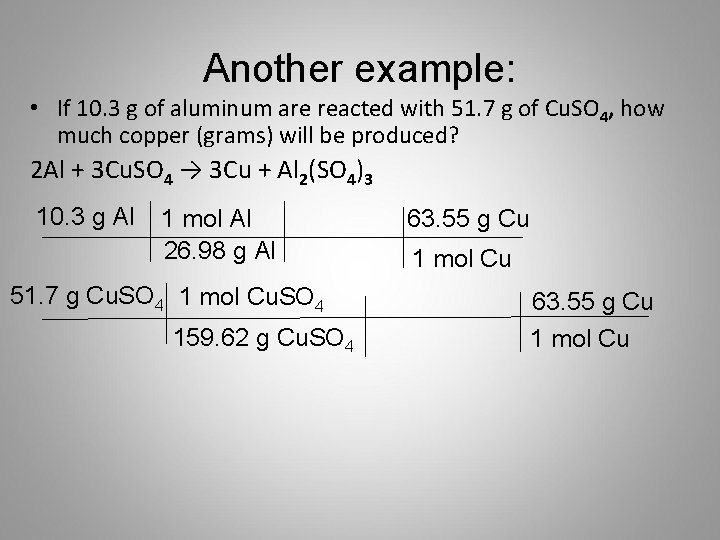
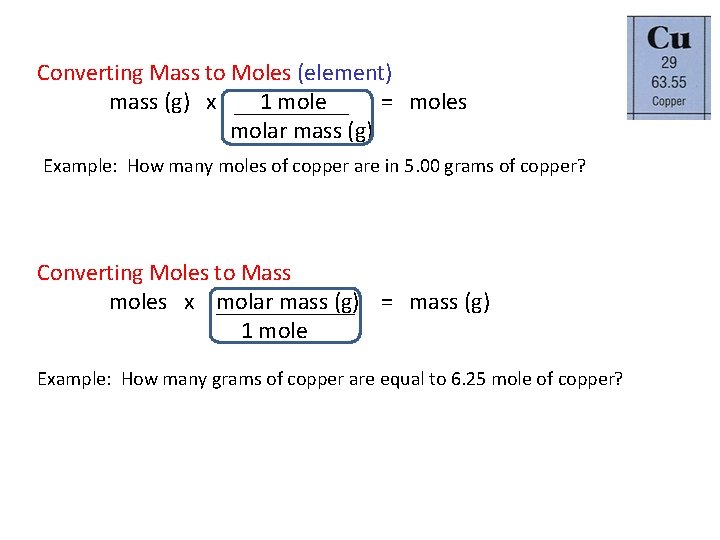
1 mole is equal to 1 moles Copper II Oxide or 795454 grams. Is the dehydration and hydration of CuSO4 5h2o reversible. 1 mole is equal to 1 moles CoPPEr I CaRbONaTe or 707179162 grams. Molecular weight of Copper or grams The molecular formula for Copper is Cu. 22 grams Cu 1 moles Cu 635 grams Cu 22 grams Cu moles Cu grams Cu Atoms or Molecules Convert using 602 x 1023 Moles. How many MOLES of copper II hydroxide are present in 205 grams of this compound.
 Source: study.com
Source: study.com
You can view more details on each measurement unit. Show transcribed image text. Molecular weight of Copper II Sulfate or grams. How Many Moles Of Cu Are In 148 Ã 1025 Cu Atoms. The SI base unit for amount of substance is the mole.
 Source: youtube.com
Source: youtube.com
1 mole is equal to 1 moles Copper or 63546 grams. How many GRAMS of copperII fluoride are present in 163 moles of this compound. Molecular weight of CuNo3 or grams The SI base unit for amount of substance is the mole. That implies 6355 gram copper contains one mole of copper that is 6355gram copper contains 6023 10 23 atoms. You can view more details on each measurement unit.
 Source: convertermaniacs.com
Source: convertermaniacs.com
1 grams CopperII Sulfate is equal to 00062653265550854 mole. There are 246 mol Cu atoms in 1481025 Cu atoms. The relation between molecular formula mass and molar mass Page 4 4 To obtain one mole of copper atoms 602 x 1023 atoms weigh out 6355 g copper. Since 1 mole of copper weights 6355 gram. In our example 16 grams 160 grams per mole 01 moles.
 Source: slidetodoc.com
Source: slidetodoc.com
Use this page to learn how to convert between moles Copper II Oxide and gram. M hydrogen 101g mol 2 202g mol. The SI base unit for amount of substance is the mole. Note that the question gave the mass as 249. You can view more details on each measurement unit.
 Source: slidetodoc.com
Source: slidetodoc.com
1 10 -6. That implies 6355 gram copper contains one mole of copper that is 6355gram copper contains 6023 10 23 atoms. The relation between molecular formula mass and molar mass Page 4 4 To obtain one mole of copper atoms 602 x 1023 atoms weigh out 6355 g copper. Likewise what is the formula for copper sulfate hydrate. M copper 6355g mol.
 Source: pinterest.com
Source: pinterest.com
Density of copper is equal to 8 940 kgm³. How do you convert moles to grams of copper. At 20C 68F or 29315K at standard atmospheric pressure. We assume you are converting between grams Copper and mole. M hydrogen 101g mol 2 202g mol.
 Source: chem.libretexts.org
Source: chem.libretexts.org
That is NA 60221023 Now apply UNITARY METHOD therefore mass of 1305 gram of Cu 60221023 1305 635 122 1023 atoms. Divide the mass of water lost when you heated the salt by the molar mass of water roughly 18 grams per mole. At 20C 68F or 29315K at standard atmospheric pressure. From your Periodic Table we learn that one mole of copper 60221023 individual copper atoms have a mass of 6355g And thus we use the MASS of a chemical sample to calculate the NUMBER of atoms and molecules. 1 mole is equal to 1 moles Copper II Oxide or 795454 grams.
 Source: slidetodoc.com
Source: slidetodoc.com
1 grams CopperII Sulfate is equal to 00062653265550854 mole. 1 000 000 000 000. Copper weighs 894 gram per cubic centimeter or 8 940 kilogram per cubic meter ie. Experts are tested by Chegg as specialists in their subject area. How many moles are in 1 mole of copper.
 Source: pinterest.com
Source: pinterest.com
Since 1 mole of copper weights 6355 gram. How many GRAMS of copperII fluoride are present in 163 moles of this compound. 22 grams Cu 1 moles Cu 635 grams Cu 22 grams Cu moles Cu grams Cu Atoms or Molecules Convert using 602 x 1023 Moles. The SI base unit for amount of substance is the mole. M oxygen 1600g mol 2 3200g mol.
 Source: slidetodoc.com
Source: slidetodoc.com
You can view more details on each measurement unit. Is the dehydration and hydration of CuSO4 5h2o reversible. How many atoms are in 22 grams of copper metal Cup If we were asked to convert 22 grams of copper to atoms of copper weld have to go from one. Since 1 mole of copper weights 6355 gram. That is NA 60221023 Now apply UNITARY METHOD therefore mass of 1305 gram of Cu 60221023 1305 635 122 1023 atoms.
 Source: slidetodoc.com
Source: slidetodoc.com
The SI base unit for amount of substance is the mole. We assume you are converting between grams CopperII Sulfate and mole. The SI base unit for amount of substance is the mole. M oxygen 1600g mol 2 3200g mol. We can do this by adding the molar masses of copper oxygen and hydrogen together accounting for the number of atoms for each element.
 Source: slidetodoc.com
Source: slidetodoc.com
How Many Moles Of Cu Are In 148 Ã 1025 Cu Atoms. There are 246 mol Cu atoms in 1481025 Cu atoms. How many moles of Cu are in Cu atoms. We assume you are converting between grams Copper and mole. We assume you are converting between grams CopperII Sulfate and mole.
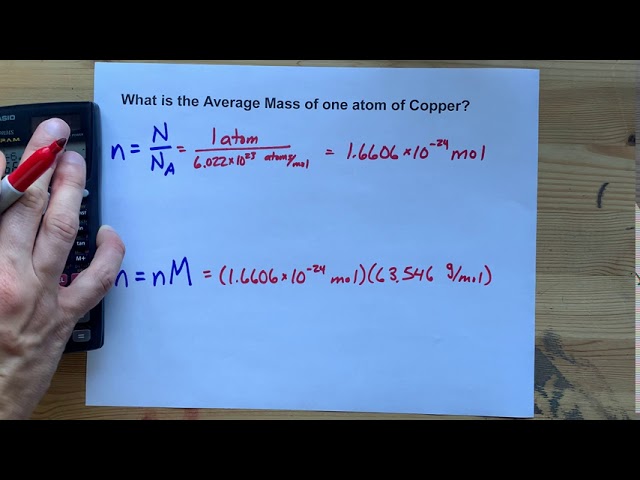 Source: youtube.com
Source: youtube.com
Use this page to learn how to convert between moles Copper II Oxide and gram. Since 1 mole of copper weights 6355 gram. The molar mass M of a substance is the mass of one mole of entities atoms molecules or formula units of the substance. Answer 1 of 4. Note that the question gave the mass as 249.
 Source: slidetodoc.com
Source: slidetodoc.com
Molecular weight of Copper or mol The molecular formula for Copper is Cu. 22 grams Cu 1 moles Cu 635 grams Cu 22 grams Cu moles Cu grams Cu Atoms or Molecules Convert using 602 x 1023 Moles. Answer 1 of 4. Number of moles in 249 g of CuSO4. The SI base unit for amount of substance is the mole.
 Source: slidetodoc.com
Source: slidetodoc.com
Answer 1 of 4. That implies 6355 gram copper contains one mole of copper that is 6355gram copper contains 6023 10 23 atoms. 1023 individual copper atoms have a mass of 6355 g. Divide the mass of your anhydrous heated salt sample by the molar mass of the anhydrous compound to get the number of moles of compound present. We assume you are converting between grams CopperII Sulfate and mole.
This site is an open community for users to submit their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site value, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title how many moles are in 1 gram of copper by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






