Your How many moles are in 1 gram of calcium images are available. How many moles are in 1 gram of calcium are a topic that is being searched for and liked by netizens today. You can Find and Download the How many moles are in 1 gram of calcium files here. Find and Download all free photos.
If you’re looking for how many moles are in 1 gram of calcium images information linked to the how many moles are in 1 gram of calcium interest, you have pay a visit to the right site. Our site frequently gives you suggestions for seeing the highest quality video and picture content, please kindly hunt and locate more enlightening video content and images that match your interests.
How Many Moles Are In 1 Gram Of Calcium. No of moles 9840. Atomic mass 40 gmol. That is equal to 0132560231023 7981022 atoms of calcium. How many grams are in 1 mole of calcium.
 Pin On Healing From pinterest.com
Pin On Healing From pinterest.com
Calculate the number of moles present in. You can view more details on each measurement unit. 6022 x 10. No of moles 9840. 2384 g of Ca 140 2384 0596 moles. The molar mass is defined as the grams of compound present in one mole.
No of moles of Calcium 0245 mol.
Note that rounding errors may occur so always check the results. As we know that one mole is equal to the one gram atom which is also equal to the molar mass. The SI base unit for quantity of substance is the mole. The molar mass of calcium is 40 gmole. Atomic mass 40 gmol. Use this page to learn how to.
 Source: youtube.com
Source: youtube.com
1 mol of Ca 40 grams. Therefore the mass of 15 gram atom of calcium is 60 gram. Use this web page to discover ways to convert between moles Calcium and gram. Atomic mass 40 gmol. The molar mass of calcium nitrate is 1640878 gmol.
 Source: slideplayer.com
Source: slideplayer.com
Number of oxygen atoms. First we need to know how many grams are in 1 mole of calcium. 390 Unit 6 Changes in Matter The formula mass of CaCO3 is 10009 amu and therefore the molar mass of one mole of CaCO3 is 10009 grams. How many moles Calcium Iodide in 1 grams. Note that rounding errors may occur so always check the results.
 Source: toppr.com
Source: toppr.com
Thus number of moles in 25 g of calcium carbonate is 025. Use the molecular formula to find the molar mass. 6022 x 10. No of moles of Calcium 0245 mol. Use this web page to discover ways to convert between moles Calcium and gram.
 Source: youtube.com
Source: youtube.com
Use this web page to discover ways to convert between moles Calcium and gram. How many moles Calcium Iodide in 1 grams. The SI base unit for amount of substance is the mole. The molar mass of calcium nitrate is 1640878 gmol. The molar mass of calcium is 40 gmole.
 Source: pinterest.com
Source: pinterest.com
Use this page to learn how to convert between moles Calcium Oxide and gram. Use this page to learn how to. The molar mass of calcium is 40 gmole. Calcium is 40 g so 1 g contains 6023 x 102340 atoms 1506 x Use this page to learn how to convert between grams Calcium Chloride and mole. Type in your own numbers in the form to convert the units.
 Source: pinterest.com
Source: pinterest.com
Atomic mass 40 gmol. 1 grams Calcium is equal to 0024951344877489 mole. No of moles of Calcium 0245 mol. 390 Unit 6 Changes in Matter The formula mass of CaCO3 is 10009 amu and therefore the molar mass of one mole of CaCO3 is 10009 grams. How many moles Calcium Iodide in 1 grams.
 Source: toppr.com
Source: toppr.com
As we know that one mole is equal to the one gram atom which is also equal to the molar mass. The molar mass of calcium nitrate is 1640878 gmol. Molecular weight of Calcium or grams. How many moles Calcium Iodide in 1 grams. Use this web page to discover ways to convert between moles Calcium and gram.
 Source: pinterest.com
Source: pinterest.com
To find the number of moles in 98g of calcium well just divide 4008 by 98. For example one mole of calcium contains. Number of moles Gram weight Atomic weight of substance We know that Atomic weight of calcium Ca is 40078 u Number of moles in 205 grams of calcium Ca Amount of calcium Ca Atomic weight of calcium Ca. If you dissolved 107 g ofNH4Cl in water how many 1 moles of ions would be in solution. 390 Unit 6 Changes in Matter The formula mass of CaCO3 is 10009 amu and therefore the molar mass of one mole of CaCO3 is 10009 grams.

Molecular weight of Calcium or grams. Answered 11 months ago Author has 70 answers and 238K answer views. For example one mole of calcium contains. Multiply moles of Ca by the conversion factor molar mass of calcium 4008 g Ca 1 mol Ca which then allows the cancelation of moles leaving grams of Ca. We assume you are converting between moles Calcium Iodide and gram.

Molecular weight of Calcium Iodide or grams The molecular formula for Calcium Iodide is CaI2. The SI base unit for amount of substance is the mole. How many moles Calcium Iodide in 1 grams. Note that rounding errors may occur so always check the results. Atomic mass of calcium 40 u.
 Source: youtube.com
Source: youtube.com
The total number of atoms in a substance can also be determined by using the relationship between grams moles and atoms. Calculate the number of moles present in. 1 mol of Ca 40 grams. What is the total amount of moles of atoms in ajar that contains 241 x 10 atoms of chromium 151 x 10 atoms of nickel and 301 x 1023 atoms of copper. The number of moles are calculated as shown below.

Answered 11 months ago Author has 70 answers and 238K answer views. 1501023 atoms a10-23 atomsROUGH WORK. 390 Unit 6 Changes in Matter The formula mass of CaCO3 is 10009 amu and therefore the molar mass of one mole of CaCO3 is 10009 grams. Use this page to learn how to. For example one mole of calcium contains.
 Source: instasolv.com
Source: instasolv.com
Number of moles Gram weight Atomic weight of substance We know that Atomic weight of calcium Ca is 40078 u Number of moles in 205 grams of calcium Ca Amount of calcium Ca Atomic weight of calcium Ca. 1 g 140 mol of Ca. This is equivalent to 0132560231023 7981022 atoms of calcium. First we need to know how many grams are in 1 mole of calcium. How many moles Calcium Iodide in 1 grams.
 Source: youtube.com
Source: youtube.com
NH4 and one chloride ion CFI. 1 mol of Ca 40 grams. Put it in formula. Use this page to learn how to convert between moles Calcium Oxide and gram. Thus number of moles in 25 g of calcium carbonate is 025.
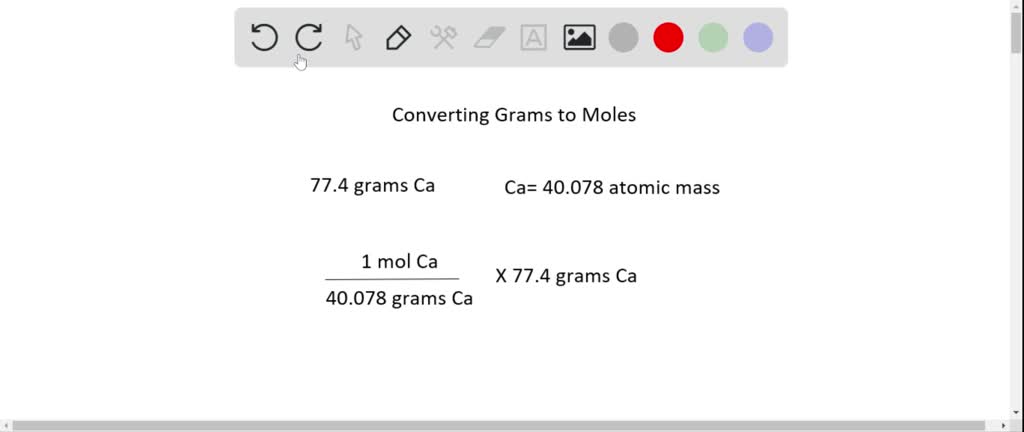 Source: numerade.com
Source: numerade.com
That is equal to 0132560231023 7981022 atoms of calcium. Number of moles Gram weight Atomic weight of substance We know that Atomic weight of calcium Ca is 40078 u Number of moles in 205 grams of calcium Ca Amount of calcium Ca Atomic weight of calcium Ca. How many moles are 5 grams of calcium. Use this page to learn how to convert between moles Calcium and gram. Note that rounding errors may occur so always check the results.
This site is an open community for users to share their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site serviceableness, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title how many moles are in 1 gram of calcium by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






