Your How many moles are contained in 237 g of water 1 cup images are available. How many moles are contained in 237 g of water 1 cup are a topic that is being searched for and liked by netizens now. You can Find and Download the How many moles are contained in 237 g of water 1 cup files here. Get all royalty-free photos and vectors.
If you’re searching for how many moles are contained in 237 g of water 1 cup pictures information related to the how many moles are contained in 237 g of water 1 cup keyword, you have visit the ideal site. Our site frequently gives you suggestions for seeking the highest quality video and image content, please kindly hunt and locate more enlightening video articles and graphics that fit your interests.
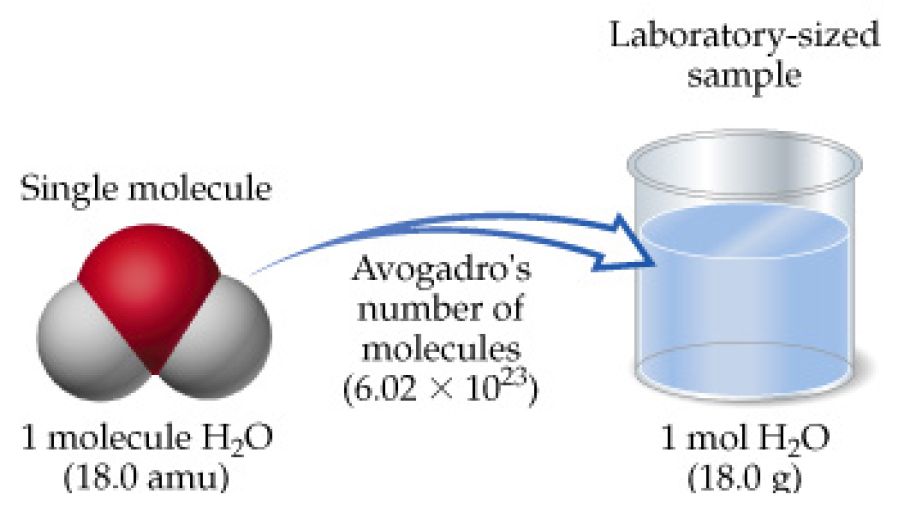
How Many Moles Are Contained In 237 G Of Water 1 Cup. The molecular formula for Tin is Sn. M 6 l 998 kgm³ 0006 m³ 998 kgm³ 5988 kg. The density of water is 1 gram per cc so 1 mole of water takes 18cc of volume. Each H 2O molecule contains 2 H atoms and 1 O atom Each mole of H 2O molecules contains 2 moles of H and 1 mole of O One mole of O atoms corresponds to 159994 g Two moles of H atoms corresponds to 2 x 10079 g Sum molar mass 180152 g H 2O per mole Chapter 3 Calculation of Molar Masses Calculate the molar mass of the following.
 How Many Molecules Are In 48 90 Grams Of Water Socratic From socratic.org
How Many Molecules Are In 48 90 Grams Of Water Socratic From socratic.org
The density of water is 1 gram per cc so 1 mole of water takes 18cc of volume. 100 1 rating 2E 793 x 10 24 3. A 3 mole of N2reacts with 9 moles of H2. 250cc - so 1 cup will contain approximately 1389 moles of water. A 132 moles b 4270 moles c 00760 moles d. You can view more details on each measurement unit.
One mole 6022x1023 molecules occupies 18ml of volume.
The molecular formula for Tin is Sn. The temperature of the mixture rises from 221 ºC to 370. Tin was among the the first metals used by humans. 18 g of H2O 1 mole 180 g of H2O X X 18018 10 moles. The specific heat of the resulting solution is 390 JC1g1. 12 gmol How many moles of oxygen are in 12 g of CO 2.
 Source: mdpi.com
Source: mdpi.com
Since 1 gram 1ml of water we can see that a cup of water contains about 1372 moles of water atoms or 826 x 1024 molecules of water. 313 1028photons LONG ANSWER. The molarity of a solution that contains 80 g of NaOH in a liter of solution is. 29 How many molecules are in 237 g about a cup of water. How many molecules are in 237 g about a cup of water.
 Source: studylib.net
Source: studylib.net
The specific heat of the resulting solution is 390 JC1g1. The cup is rounded to precisely 240 mL by US federal regulations FDA for food labeling purposes. Tin was among the the first metals used by humans. 1 mole is equal to 1 moles Tin or 11871 grams. The molarity of a solution that contains 050 moles of NaOH in 2000 milliliters of water is.
 Source: chegg.com
Source: chegg.com
In a calorimetry experiment 500 g of a 204 molekg HCl solution is mixed with 500 g of a 213 molkg NaOH solution. 18 g of H2O 1 mole 180 g of H2O X X 18018 10 moles. How many molecules are in 237 g about a cup of water. 5988 kg 5988 g. This is the typical heat capacity of water.
 Source: clutchprep.com
Source: clutchprep.com
15131 4267 602 times 1023 93 Times 1024 793 times 10 24 Nitroglycerin has a formula C3Hs N033. The molecular formula for Tin is Sn. In the reaction CH4 2 O2 – CO2 2 H2O how many moles of oxygen are required to burn 80. The temperature of the mixture rises from 221 ºC to 370. The SI base unit for amount of substance is the mole.

Atoms example How many atoms of Cl are in 043 mols of NaCl. 15131 4267 602 times 1023 93 Times 1024 793 times 10 24 Nitroglycerin has a formula C3Hs N033. How many molecules are in 237 g about a cup of water. 100 1 rating 2E 793 x 10 24 3. 12 gmol How many moles of oxygen are in 12 g of CO 2.
 Source: chegg.com
Source: chegg.com
Nitroglycerin has a formula C3H5NO33. If you have problems with the units feel free to use our temperature conversion or. The molar mass of nitroglycerin is. M 6 l 998 kgm³ 0006 m³ 998 kgm³ 5988 kg. 12g CO 2 X 2 atoms O 055mols 44 gmol 1 molecule CO 2.

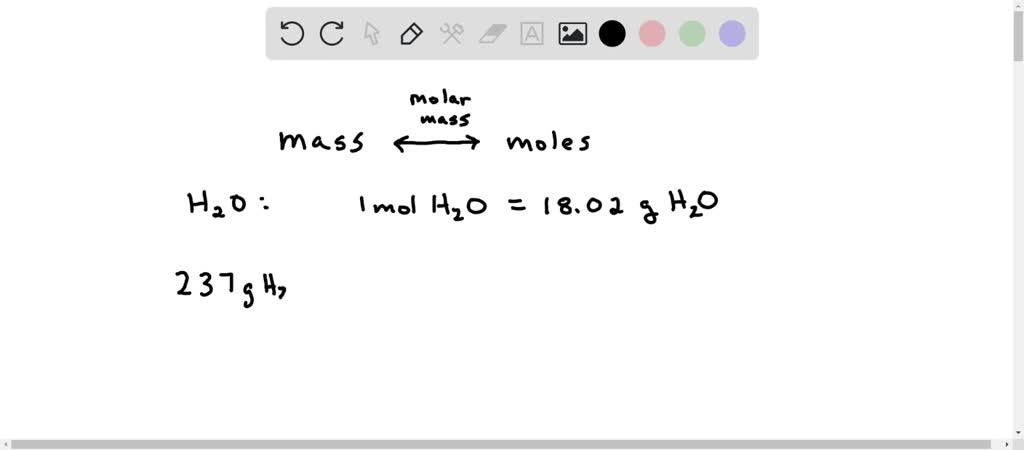
Thus the number of moles in 180 g of H2O is 10 moles. Given weight of water is 237 g. Which contains aluminum oxide Al 2 O 3 by reaction with carbon. N2 3 H2 2 NH3 Which of the following statements is NOT true for this equation. How many molecules are in 237 g about a cup of water.

The molarity of a solution that contains 80 g of NaOH in a liter of solution is. This is the typical heat capacity of water. While these are something of an approximation it is close enough for government work. How many molecules are in 237 g about a cup of water. The density of water is 1 gram per cc so 1 mole of water takes 18cc of volume.

The SI base unit for amount of substance is the mole. We assume you are converting between moles Tin and gram. How many molecules are in 237 g about a cup of water. How many water molecules are in a cup. 1 mole is equal to 1 moles Tin or 11871 grams.
 Source: studylib.net
Source: studylib.net
1 cup is approximately 250ml ie. Why is the water wet. Weight of 1 milliliter ml of pure water at temperature 4 C 1 gram g. You can also use it to find out how many moles are in a cup of water. 313 1028photons LONG ANSWER.
 Source: numerade.com
Source: numerade.com
A 3 mole of N2reacts with 9 moles of H2. While these are something of an approximation it is close enough for government work. This means that your sample contains 145 colorredcancelcolorblackmL 1 g1colorredcancelcolorblackmL 145 g. This is the typical heat capacity of water. How many moles are in 237 g about a cup of H 2 O.

1 cup is approximately 250ml ie. This is the typical heat capacity of water. Why is the water wet. We assume you are converting between moles Tin and gram. The molarity of a solution that contains 80 g of NaOH in a liter of solution is.
 Source: numerade.com
Source: numerade.com
For starters you need to know the mass of water present in your sample. A 132 moles b 4270 moles c 00760 moles d. Our 2366 grams of water divided by 18 grams per mole 1314 moles of water Our 1314 moles of water602 x 1023 molecules per mole 791 x 1024 molecules in the 8 ounce glass of water. Calculate specific heat as c Q mΔT. For every 1 mol there are 602 1023 molecules.
 Source: socratic.org
Source: socratic.org
While these are something of an approximation it is close enough for government work. The molar mass of nitroglycerin is. The answer is 00084238901524724. 12 gmol How many moles of oxygen are in 12 g of CO 2. Each H 2O molecule contains 2 H atoms and 1 O atom Each mole of H 2O molecules contains 2 moles of H and 1 mole of O One mole of O atoms corresponds to 159994 g Two moles of H atoms corresponds to 2 x 10079 g Sum molar mass 180152 g H 2O per mole Chapter 3 Calculation of Molar Masses Calculate the molar mass of the following.

How many molecules are in 237 g about a cup of water. 100 1 rating 2E 793 x 10 24 3. The temperature of the mixture rises from 221 ºC to 370. A 132 moles b 4270 moles c 00760 moles d. 250cc so 1 cup will contain approximately 1389 moles of water which will contain approximately 836 x 1024 molecules of water.
This site is an open community for users to share their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site value, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title how many moles are contained in 237 g of water 1 cup by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.





