Your How many moles are 5 grams of calcium images are ready. How many moles are 5 grams of calcium are a topic that is being searched for and liked by netizens today. You can Find and Download the How many moles are 5 grams of calcium files here. Find and Download all royalty-free vectors.
If you’re looking for how many moles are 5 grams of calcium pictures information connected with to the how many moles are 5 grams of calcium keyword, you have visit the ideal blog. Our website always provides you with suggestions for seeking the highest quality video and picture content, please kindly hunt and locate more informative video content and graphics that fit your interests.
How Many Moles Are 5 Grams Of Calcium. Therefore atoms present in 4117 mol of calcium are as follows. This compound is also known as Magnesium Chloride. The molar mass of CaO is 5607 grams per mole. 1 mole is equal to 1 moles MgCl2 or 95211 grams.
 If 100 Grams Of Calcium Carbonate Whether In The Form Of Marble Or Chalk Are Decomposed Complete Youtube From youtube.com
If 100 Grams Of Calcium Carbonate Whether In The Form Of Marble Or Chalk Are Decomposed Complete Youtube From youtube.com
We know that Atomic weight of calcium Ca is 40078 u. Step by step solution by experts to help you in doubt clearance scoring excellent marks in exams. How many calcium atoms are there in 1 mole of calcium. 2384 g of Ca 140 2384 0596. Of moles of oxygen atoms in 25 moles 625 15 Mass of 1 mole of oxygen atoms. 1 grams Calcium is equal to 0024951344877489 mole.
Thus there are 0125 mole in 5 grams of calcium.
We assume you are converting between grams Calcium and mole. Kind in your individual numbers within the kind to transform the models. Step by step solution by experts to help you in doubt clearance scoring excellent marks in exams. Step by step solution by experts to help you in doubt clearance scoring excellent marks in exams. By using the molar mass we can determine the moles of CaO present in the given sample. The SI base unit for amount of substance is the mole.
 Source: pinterest.com
Source: pinterest.com
How many moles are in 5 grams. By using the molar mass we can determine the moles of CaO present in the given sample. Do a quick conversion. If playback doesnt begin shortly try restarting your device. Molecular weight of Calcium or mol.
 Source: toppr.com
Source: toppr.com
1 moles Calcium to grams 40078 grams. 3 moles Calcium Chloride to grams 332952 grams. There are 6023 x 1023 atoms in each mole of calcium. 1 grams Calcium is equal to 0024951344877489 mole. Feb 09 2020 53 g of calcium comprises 01325 moles of calcium.
 Source: doubtnut.com
Source: doubtnut.com
3910 grams is the molar mass of one mole of K. Multiply moles of Ca by the conversion factor molar mass of calcium 4008 g Ca 1 mol Ca which then allows the cancelation of moles leaving grams of Ca. How many moles are 5 grams of calcium. 256 x 10 24 g b. Atomic mass 40 gmol.
 Source: youtube.com
Source: youtube.com
5 292215 g Molar mass of AgNO3 is 169873 2 kg AgNO3 is the same as what number of moles. The molecular formula for Calcium is Ca. 1 mole is equal to 1 moles MgCl2 or 95211 grams. Molecular weight of Calcium or mol. The answer is 40078.
 Source: pinterest.com
Source: pinterest.com
256 x 10 24 g b. The answer is 40078. 1 mole is equal to 1 moles MgCl2 or 95211 grams. The SI base unit for amount of substance is the mole. There are 6023 x 1023 atoms in each mole of calcium.
 Source: toppr.com
Source: toppr.com
How many moles are in 5 grams. The answer is 0010502988100114We assume you are converting between moles MgCl2 and gram. 801 g Ca 1 mole Ca4008 g Ca 19985 moles. Number of moles in 205 grams of calcium Ca Amount of calcium Ca Atomic weight of calcium Ca Number of moles in 205 grams of calcium Ca 205 40078. Quick conversion chart of moles Calcium to grams.
 Source: youtube.com
Source: youtube.com
The answer is 40078. 1 mol of Ca 40 grams. How Many Moles Of Sodium Bromide Can Be Made From 5 Moles Of Calcium. In the above case mass of element is 5 grams and molar mass of element is 40gmol. 1 moles Calcium to grams 40078 grams.
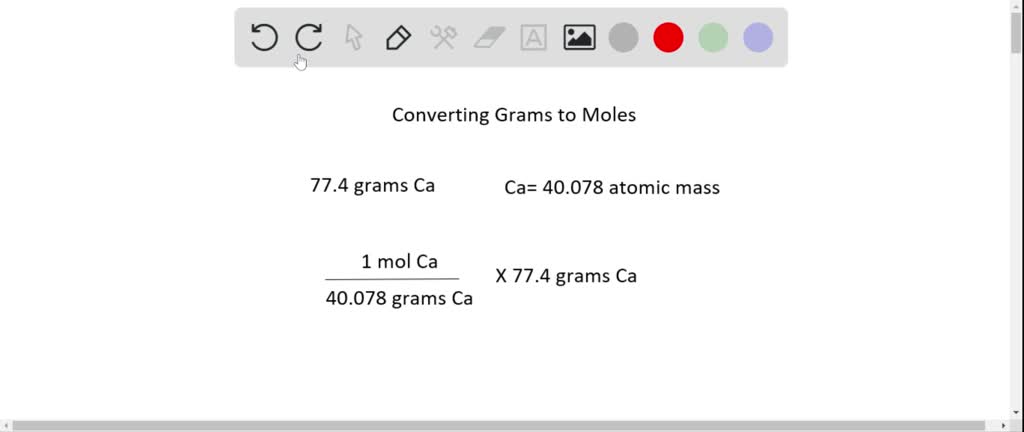 Source: numerade.com
Source: numerade.com
Molecular weight of Sodium Bromide or mol. The molecular formula for Calcium is Ca. This compound is also known as Magnesium Chloride. Answer 1 of 5. How many moles are in 5 grams.
 Source: youtube.com
Source: youtube.com
Multiply moles of Ca by the conversion factor molar mass of calcium 4008 g Ca 1 mol Ca which then allows the cancelation of moles leaving grams of Ca. No of moles 9840. Number of moles in 205 grams of calcium Ca 0511. Atomic mass of calcium 40 u. We assume you are converting between grams Calcium Bromide and mole.
 Source: youtube.com
Source: youtube.com
No of moles 9840. Molecular weight of Sodium Bromide or mol. Thus 5 grams calcium constitute 0125 mole of calcium. How many moles are 5 grams of calcium. Multiply moles of Ca by the conversion factor molar mass of calcium 4008 g Ca 1 mol Ca which then allows the cancelation of moles leaving grams of Ca.
 Source: brainly.in
Source: brainly.in
By using the molar mass we can determine the moles of CaO present in the given sample. How many grams are in 1 mole of calcium. 1 grams Calcium Carbonate 00099913175450533 mole using the molecular weight calculator and the molar mass of CaCO3. 3910 grams is the molar mass of one mole of K. 1 grams Calcium is equal to 0024951344877489 mole.
 Source: pinterest.com
Source: pinterest.com
2331 moles are in 23 grams or 007419354838 moles. You can view more details on each measurement unit. This compound is also known as Magnesium Chloride. 2331 moles are in 23 grams or 007419354838 moles. The molecular formula for Calcium is Ca.
 Source: brainly.in
Source: brainly.in
How many grams is in 1000 moles of calcium CaApplications of the Mole. Kind in your individual numbers within the kind to transform the models. We assume you are converting between grams Calcium and mole. Step by step solution by experts to help you in doubt clearance scoring excellent marks in exams. There are 6023 x 1023 atoms in each mole of calcium.
 Source: pinterest.com
Source: pinterest.com
Feb 09 2020 53 g of calcium comprises 01325 moles of calcium. The molecular formula for Calcium is Ca. Of oxygen atoms in 1 formula unit 6 No. Atomic mass 40 gmol. 22K views View upvotes.
 Source: youtube.com
Source: youtube.com
Thus 5 grams calcium constitute 0125 mole of calcium. Grams can be canceled leaving the moles of K. No of moles 9840. Feb 09 2020 53 g of calcium comprises 01325 moles of calcium. The molecular formula for Calcium Bromide is CaBr2.
This site is an open community for users to do submittion their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site beneficial, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title how many moles are 5 grams of calcium by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






