Your How many molecules in one mole of glucose images are ready. How many molecules in one mole of glucose are a topic that is being searched for and liked by netizens today. You can Download the How many molecules in one mole of glucose files here. Get all royalty-free photos and vectors.
If you’re searching for how many molecules in one mole of glucose pictures information linked to the how many molecules in one mole of glucose interest, you have come to the right blog. Our site frequently provides you with suggestions for seeing the highest quality video and image content, please kindly search and locate more informative video content and graphics that match your interests.
How Many Molecules In One Mole Of Glucose. Then the number of molecules that could be generated from one glucose molecule could be calculated by dividing the total energy by energy of one mole of ATP ie. Therefore 4 moles of glucose contains 4 x 6022 x 1023 molecules. There are 51171021 molecules present in 153g of C6H12O6 glucose. Which equates to about 3011 x 1024 molecules of glucose per 5 moles of glucose.
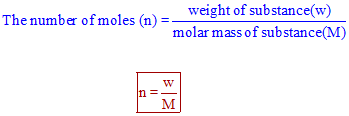 Number Of Moles Of Glucose Molecules From adichemistry.com
Number Of Moles Of Glucose Molecules From adichemistry.com
Number of carbon atoms in 1 mole of glucose 6 x 6022 x 1023 atoms. 100 1 rating Sol - 1 From first question we have to find molecules of glucose present in 18 moles of glucose as follows - We have know that 1 mole glucose or any molecule contains 6022 1023 molecules of glucose. One mole of glucose molecule has a mass of 18016 g. Glucose has a molar mass of 18016 gmol. How many atoms are in a molecule of CO2. Therefore your 90-g sample will contain 90g 1 mole C6H12O6 180156g 04996 moles C5H12O6 As you know the number of molecules you get per mole is given by Avogadros number.
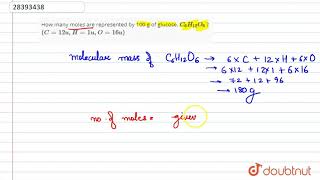
Molar mass of C6H12O66x12 12x1 6x16 72 12 96 180 g mol-1 Now we can calculate the number of moles of glucose as follows.
How many molecules are present in a mole of glucose C6H12O6. So the number of molecules present will be. 1 5 moles A 1 8 m o l e s of B and 2 0 m o l e s of C reacted together according to the reaction A 2 B 4 C A B 2 C 4. 692 x 10 -26 c. The molar mass of a substance is the mass of one mole of the substance. Similarly how many molecules are in a mole.
 Source: pinterest.com
Source: pinterest.com
This will act as the conversion factor that will take you from moles to molecules. This gives an answer of 57 ATP. Number of carbon atoms in 1 molecule of glucose 6. 1 mole of glucose contains 6022 x 1023 molecules. Molar mass of C6H12O66x12 12x1 6x16 72 12 96 180 g mol-1 Now we can calculate the number of moles of glucose as follows.

How many moles of hydrogen atoms are contained in a mole of glucose. This gives an answer of 57 ATP. One mole of glucose molecule has a mass of 18016 g. Then the number of molecules that could be generated from one glucose molecule could be calculated by dividing the total energy by energy of one mole of ATP ie. 1 mole of glucose contains 6022 x 1023 molecules.
 Source: youtube.com
Source: youtube.com
Of glucose molecules 001 x 6022 x 10 23 6022 x 10 21 Remember that one mole of any substance contains Avogadro number of particles may be atoms or molecules or ions etc. Similarly how many molecules are in a mole. 6022xx1023 mol-1 N_A. How many moles of hydrogen atoms are contained in a mole of glucose. So the number of molecules present will be.
 Source: adichemistry.com
Source: adichemistry.com
1 molecule of glucose contains 6 atoms of C 12 atoms of H and 6 atoms of O 1 mole of glucose contains 6 moles of C atoms 12 moles of H atoms and 6 moles of O atoms. 12 Moles of hydrogenHow many moles of oxygen and hydrogen are in one mole of H2O containsOne mole of oxygen atoms Two moles of hydrogen atomsMole Concepts A mole of glucose C6H12O6 contains 6022 X 1023molecules of glucose. The molar mass of a substance is the mass of one mole of the substance. 241 x 1024 molecules. 1 5 moles A 1 8 m o l e s of B and 2 0 m o l e s of C reacted together according to the reaction A 2 B 4 C A B 2 C 4.
 Source: pinterest.com
Source: pinterest.com
Which equates to about 3011 x 1024 molecules of glucose per 5 moles of glucose. The molar mass of glucose is 6xx1201112xx107946xx1599gmol-1. So in 1 mol there are Avogadros number of molecules ie. 1 mole 60221023 6022 10 23 atoms molecules protons. Therefore 4 moles of glucose contains 4 x 6022 x 1023 molecules.

Simply as a dozen eggs is 12 eggs a mole of glucose is 6021023 glucose molecules and a mole of carbon atoms is 6021023 carbon atoms. There are 00085 or 85103 moles present. Number of carbon atoms in 1 molecule of glucose 6. This gives an answer of 57 ATP. Click to see full answer.
 Source: toppr.com
Source: toppr.com
One mole of glucose molecule has a mass of 18016 g. Simply as a dozen eggs is 12 eggs a mole of glucose is 6021023 glucose molecules and a mole of carbon atoms is 6021023 carbon atoms. 10079 12120107 6159994 6 18016 gmol. So altogether the molar mass of a single molecule of glucose is equal to. 1 mole 60221023 6022 10 23 atoms molecules protons.
 Source: sciencetrends.com
Source: sciencetrends.com
One mole of glucose molecule has a mass of 18016 g. Hence there are 3011 10 23 molecules in 05moles of glucose. Glucose has a molar mass of 180156 gmol which means that one mole of glucose molecules has a mass of 180156 g. Therefore 4 moles of glucose contains 4 x 6022 x 1023 molecules. Glucose has a molar mass of 18016 gmol.

692 x 10 -26 c. N S 05 6022 1 0 23 N S 3011 10 23. Click to see full answer. In one molecule of glucose there are 12 hydrogen 6 carbon and 6 oxygen atoms. This number is 6022 x 1023 molecules per mole of a substance.
 Source: numerade.com
Source: numerade.com
Similarly how many molecules are in a mole. In one molecule of glucose there are 12 hydrogen 6 carbon and 6 oxygen atoms. 692 x 10 -26 c. Therefore the correct option is A Fifty Seven. The molar mass of a substance is the mass of one mole of the substance.
 Source: youtube.com
Source: youtube.com
One mole of glucose molecule has a mass of 18016 g. How many moles of hydrogen atoms are contained in a mole of glucose. Hence there are 3011 10 23 molecules in 05moles of glucose. A692 x10 26 B334 x 10 21 C144 x 10 25 D602 x 10 23. Therefore the correct option is A Fifty Seven.
 Source: pinterest.com
Source: pinterest.com
Molar mass of C6H12O66x12 12x1 6x16 72 12 96 180 g mol-1 Now we can calculate the number of moles of glucose as follows. There are 00085 or 85103 moles present. How many atoms are in a molecule of CO2. Molar mass of C6H12O66x12 12x1 6x16 72 12 96 180 g mol-1 Now we can calculate the number of moles of glucose as follows. Of glucose molecules 001 x 6022 x 10 23 6022 x 10 21 Remember that one mole of any substance contains Avogadro number of particles may be atoms or molecules or ions etc.
 Source: nl.pinterest.com
Source: nl.pinterest.com
Therefore 4 moles of glucose contains 4 x 6022 x 1023 molecules. This number is 6022 x 1023 molecules per mole of a substance. Glucose is C6H12O6 and Avogadros Number named for Amadeo Carlo Avogadro 1776 1856 tells us that 1 mole contains 6022 x 1023 molecules. So in 1 mol there are Avogadros number of molecules ie. One mole of glucose molecule has a mass of 18016 g.
 Source: youtube.com
Source: youtube.com
Simply as a dozen eggs is 12 eggs a mole of glucose is 6021023 glucose molecules and a mole of carbon atoms is 6021023 carbon atoms. The answer is rounded to three sig figs the number of sig figs you have for the number of moles of glucose. The chemical formula for glucose is C6H12O6 C 6 H 12 O 6. 1 mole of glucose contains 6022 x 1023 molecules. One mole is 6021023 molecules.
 Source: slideplayer.com
Source: slideplayer.com
1 5 moles A 1 8 m o l e s of B and 2 0 m o l e s of C reacted together according to the reaction A 2 B 4 C A B 2 C 4. 241 x 1024 molecules. A692 x10 26 B334 x 10 21 C144 x 10 25 D602 x 10 23. 1 molecule of glucose contains 6 atoms of C 12 atoms of H and 6 atoms of O 1 mole of glucose contains 6 moles of C atoms 12 moles of H atoms and 6 moles of O atoms. 1 mole of glucose contains 6022 x 1023 molecules.
This site is an open community for users to share their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site value, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title how many molecules in one mole of glucose by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






