Your How many molecules in a gram of sugar images are available in this site. How many molecules in a gram of sugar are a topic that is being searched for and liked by netizens now. You can Get the How many molecules in a gram of sugar files here. Download all free vectors.
If you’re searching for how many molecules in a gram of sugar pictures information related to the how many molecules in a gram of sugar interest, you have come to the ideal site. Our site always provides you with suggestions for seeing the highest quality video and image content, please kindly hunt and find more enlightening video articles and images that fit your interests.
How Many Molecules In A Gram Of Sugar. What is the number of molecules in 1 gram of sugar. Of atoms in 1 molecules of SugarC12 H22 O11. Then multiply that number by 6022 x 10²³ which is 1759x10²¹ molecules or 1759000000000000000000 molecules. 342 g of cane sugar will contain.
 Chapter 10 Answers Ppt Download From slideplayer.com
Chapter 10 Answers Ppt Download From slideplayer.com
Now it is a fact that in a mole of stuff there are 6022 1023 individual items of stuff. If we take atomic substance then the number of atoms in one mole is equal to Avogadro number. So the molecular weight or weight of a mole of sugar is 180g. Note that rounding errors may occur so always check the results. 1 grams Sucrose is equal to 00029214440066693 mole. The molecular weight of the sugar molecule found in cane sugar is the sum of the atomic weights of the 12 carbon atoms 22 hydrogen atoms and 11 oxygen atoms in a C12H22O11 molecule.
If a grain of sugar is 200 micrograms 00002 grams and there are 6021023 molecules in a mole Avogadros number which is the number.
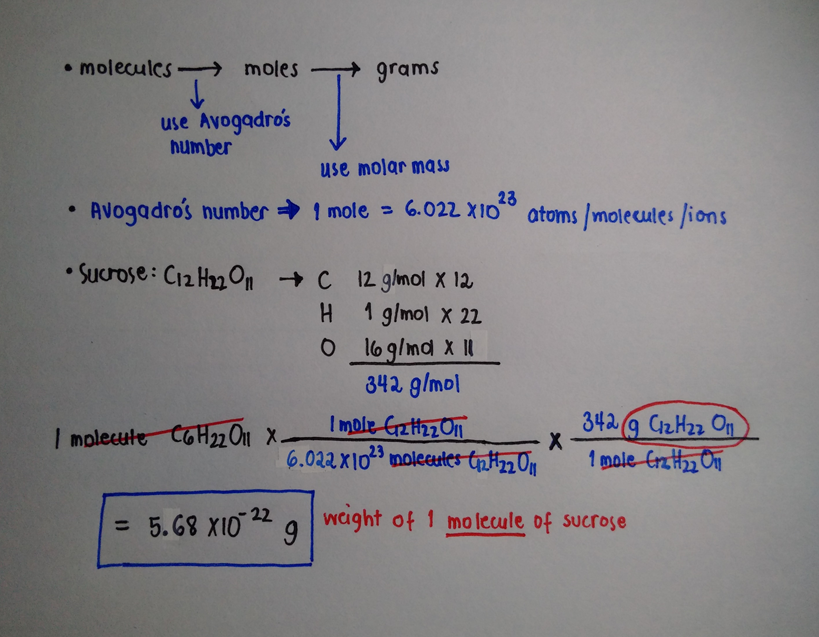
Molecular weight of Sucrose or mol. Sulfur molecule S8 133. Convert 1st Grams to Moles then Moles to Molecules Molecules of Glucose 523 g Glucose x 1 mol Glucose1800 Glucose x 602 x 1023 molecules of Glucose1 mol Glucose 175 x 1022 molecules of Glucose Convert 1st Grams to Molecules then Molecules to Atoms Atoms O 175 x 1022 molecules Glucose 6 atoms O molecule Glucose 105 x 1023 atoms O. Additionally how many moles are in AMUS. Molar mass of C 12 g mol -1. One mole 180 grams Grams of sugar 0086 moles x 180 gmole 1552 g did not use sig figs Continue reading.
 Source: slideplayer.com
Source: slideplayer.com
So 133 mol 6022 1023 mol1 8 1023 individual sucrose molecules. Convert 1st Grams to Moles then Moles to Molecules Molecules of Glucose 523 g Glucose x 1 mol Glucose1800 Glucose x 602 x 1023 molecules of Glucose1 mol Glucose 175 x 1022 molecules of Glucose Convert 1st Grams to Molecules then Molecules to Atoms Atoms O 175 x 1022 molecules Glucose 6 atoms O molecule Glucose 105 x 1023 atoms O. Of moles 1000342 292 moles. 342 g of cane sugar will contain. Molecular weight of Sucrose or mol.

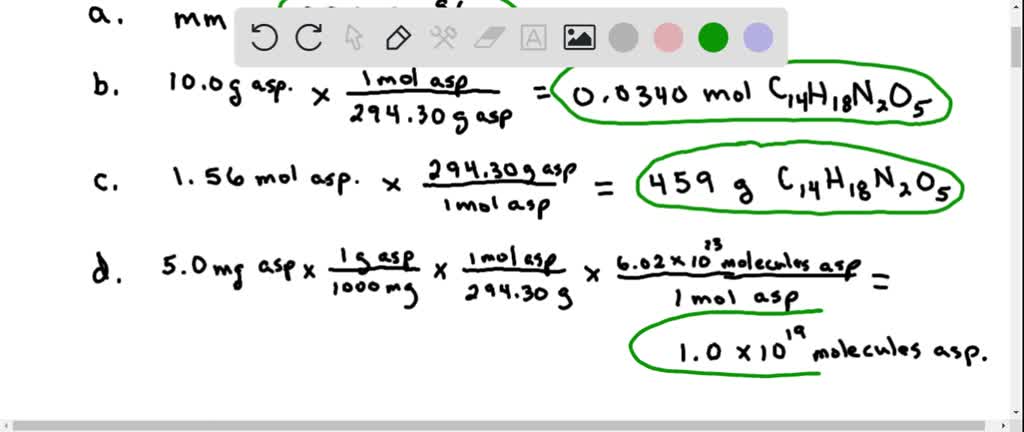
Mole To Gram Problems Since theres 15 calories in a packet or cube of sugar and each is 4 grams that means the 40 grams of sugar equals 10 packets of sugar in a can of soda. Now it is a fact that in a mole of stuff there are 6022 1023 individual items of stuff. The answer is 18015588. A sugar crystal contains approximately 171017 sucrose C12H22O11 molecules What is its mass in mg. Convert 1st Grams to Moles then Moles to Molecules Molecules of Glucose 523 g Glucose x 1 mol Glucose1800 Glucose x 602 x 1023 molecules of Glucose1 mol Glucose 175 x 1022 molecules of Glucose Convert 1st Grams to Molecules then Molecules to Atoms Atoms O 175 x 1022 molecules Glucose 6 atoms O molecule Glucose 105 x 1023 atoms O.
 Source: socratic.org
Source: socratic.org
The answer is 18015588. C12H22O11 has a molecular weight of 342299 amu. The SI base unit for amount of substance is the mole. Soobee72pl and 45 more users found this answer helpful. Moles of sucrose 454 g 34230 g mol1 133 mol.
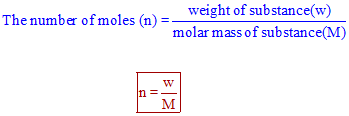 Source: adichemistry.com
Source: adichemistry.com
That is a large number in this case approximately. Since one mole is one mole irrespective of the substance a mole of sugar will not in any way be different. 612 121 116 180 grams. Of moles Avogadros number. One gram of table sugar is equal to 176 x 1021 molecules of sucrose.

Note that rounding errors may occur so always check the results. That is a large number in this case approximately. Of molecules No. One gram of table sugar is equal to 176 x 1021 molecules of sucrose. 1 grams Sucrose is equal to 00029214440066693 mole.
 Source: youtube.com
Source: youtube.com
C12H22O11 has a molecular weight of 342299 amu. Note that rounding errors may occur so always check the results. Molar mass of the Sugar 342 gmole. We assume you are converting between grams Glucose and mole. The answer is 18015588.
 Source: clutchprep.com
Source: clutchprep.com
1 mole of C12H22O11342gC12H22O11342g. Atomic weight molecular weight and molar mass This shared value between molar mass and atomic mass applies to. Of moles 1000342 292 moles. How many molecules are there in 125 grams of sugar C12H22O11 - 16608482. Also Know how many moles are in one sugar.

Mole To Gram Problems Since theres 15 calories in a packet or cube of sugar and each is 4 grams that means the 40 grams of sugar equals 10 packets of sugar in a can of soda. How Many Moles In A Gram Of Sugar. Mole To Gram Problems Since theres 15 calories in a packet or cube of sugar and each is 4 grams that means the 40 grams of sugar equals 10 packets of sugar in a can of soda. A mole of C12H22O11 would have a mass of 342299 grams. Molecular weight of Sucrose or mol.
 Source: nutritionnews.abbott
Source: nutritionnews.abbott
1 mole of C12H22O11342gC12H22O11342g. Also Know how many moles are in one sugar. Since one mole is one mole irrespective of the substance a mole of sugar will not in any way be different. The SI base unit for amount of substance is the mole. Of molecules 292 6022 10²³ 1758 10²³ molecules.
 Source: studylib.net
Source: studylib.net
Additionally how many moles are in AMUS. 12 x 12 22 x 1 11 x 16 342 amu. From the molecular formula C 6 H 12 O 6 one can find there are 6 carbon atoms 12 hydrogen atoms and 6 oxygen atoms in one molecule of glucose. 2 Calculate the number of atoms present in 54 grams of aluminium. Chlorine molecule Cl2 483.

One mole 180 grams Grams of sugar 0086 moles x 180 gmole 1552 g did not use sig figs Continue reading. Atomic weight molecular weight and molar mass This shared value between molar mass and atomic mass applies to. Molar mass of O 12 g mol -1. 612 121 116 180 grams. Moles of sucrose 454 g 34230 g mol1 133 mol.

Of molecules 292 6022 10²³ 1758 10²³ molecules. If a grain of sugar is 200 micrograms 00002 grams and there are 6021023 molecules in a mole Avogadros number which is the number. C12H22O11 has a molecular weight of 342299 amu. Of moles Avogadros number. And where one mole is equal to Avogadros numberwhich is equal to 602214076 10powerof23.
 Source: numerade.com
Source: numerade.com
You can view more details on each measurement unit. From the molecular formula C 6 H 12 O 6 one can find there are 6 carbon atoms 12 hydrogen atoms and 6 oxygen atoms in one molecule of glucose. We assume you are converting between grams Glucose and mole. 1Mole In simple term it is defined as gram solute by its molecular weight. Of moles 1000342 292 moles.
 Source: slideplayer.com
Source: slideplayer.com
The molecular weight of the sugar molecule found in cane sugar is the sum of the atomic weights of the 12 carbon atoms 22 hydrogen atoms and 11 oxygen atoms in a C12H22O11 molecule. 1 grams Glucose is equal to 0. 1Mole In simple term it is defined as gram solute by its molecular weight. Molar mass of the Sugar 342 gmole. So this is where we get the conversion between 1 mole and 6022 10 23 molecules.
 Source: slideplayer.com
Source: slideplayer.com
Note that rounding errors may occur so always check the results. How many molecules are there in 125 grams of sugar C12H22O11 - 16608482. Of molecules 292 6022 10²³ 1758 10²³ molecules. Then you need to figure out how many moles of. Now 342 g of cane sugar contain 6022102360221023 molecules.
This site is an open community for users to share their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site adventageous, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title how many molecules in a gram of sugar by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.