Your How many molecules in 1 mole of glucose images are ready. How many molecules in 1 mole of glucose are a topic that is being searched for and liked by netizens today. You can Find and Download the How many molecules in 1 mole of glucose files here. Get all royalty-free photos and vectors.
If you’re looking for how many molecules in 1 mole of glucose images information linked to the how many molecules in 1 mole of glucose interest, you have come to the right site. Our site always gives you suggestions for viewing the maximum quality video and image content, please kindly hunt and find more informative video articles and images that fit your interests.
How Many Molecules In 1 Mole Of Glucose. Molecular weight of Glucose or mol The molecular formula for Glucose is C6H12O6. There are 00085 or 85103 moles present. N S 05 6022 1 0 23 N S 3011 10 23. We assume you are converting between grams Glucose and mole.
 Atoms And Molecules Class 9 Extra Questions Science Chapter 3 Learn Cbse Extraquestionsforclass9sciencechapter3 Class9sciencenotes Class Science Molecules From cz.pinterest.com
Atoms And Molecules Class 9 Extra Questions Science Chapter 3 Learn Cbse Extraquestionsforclass9sciencechapter3 Class9sciencenotes Class Science Molecules From cz.pinterest.com
N S 05 6022 1 0 23 N S 3011 10 23. You have a milligram mass. 1201076 10079412 1599946 Percent composition by element. It is also possible to get the number of molecules in the given sample by multiplying the number of moles with Avogadro number NA the no. Convert grams Glucose to moles or moles Glucose to grams. Therefore the correct option is A Fifty Seven.
The definition of a mole that is most commonly used in HS and College chemistry classes is that 1 mole of anything is 6022 x 1023 pieces of that thing.
It is also possible to get the number of molecules in the given sample by multiplying the number of moles with Avogadro number NA the no. The chemical formula of glucose is C12H22O11 C 12 H 22 O 11. You can view more details on each measurement unit. How many atoms are in a molecule of CO2. 6022xx1023 mol-1 N_A. 400moles Avogadros number 6022 1023molec.
 Source: pinterest.com
Source: pinterest.com
The molar mass of a substance is the mass of one mole of the substance. Molar mass of C6H12O66x12 12x1 6x16 72 12 96 180 g mol-1 Now we can calculate the number of moles of glucose as follows. 1 grams Glucose is equal to 00055507486072617. Molar mass of C6H12O6 18015588 gmol.
 Source: pinterest.com
Source: pinterest.com
If the substance is molecular the number molecules will be equal to 6022 x 10 23. Well in one mole of glucose there are 60221023 individual glucose molecules. 1 12 60221023 19931023 Note about the mole. So one mole of sugar glucose - C6H12O6 is equal to 6022 x 1023 molecules of sugar. Convert 1st Grams to Moles then Moles to Molecules Molecules of Glucose 523 g Glucose x 1 mol Glucose1800 Glucose x 602 x 1023 molecules of Glucose1 mol Glucose 175 x 1022 molecules of Glucose Convert 1st Grams to Molecules then Molecules to Atoms Atoms O 175 x 1022 molecules Glucose 6 atoms O molecule Glucose 105 x 1023.
 Source: pinterest.com
Source: pinterest.com
The chemical formula of glucose is C12H22O11 C 12 H 22 O 11.
 Source: za.pinterest.com
Source: za.pinterest.com
The answer is 18015588. The molar mass of a substance is the mass of one mole of the substance. Well in one mole of glucose there are 60221023 individual glucose molecules. You probably have one molecule of glucose it weighs 180 amu or thereabouts. 400moles Avogadros number 6022 1023molec.
 Source: pinterest.com
Source: pinterest.com
You probably have one molecule of glucose it weighs 180 amu or thereabouts. 05 moleliter x 1 liter x 6023x1023 moleculesmole 3012x1023 molecules What is the concentration of the 05M glucose solution expressed in. 400moles Avogadros number 6022 1023molec. Of glucose molecules 001 x 6022 x 10 23 6022 x 10 21 Remember that one mole of any substance contains Avogadro number of particles may be atoms or molecules or ions etc. There are 51171021 molecules present in 153g of C6H12O6 glucose.
 Source: pinterest.com
Source: pinterest.com
Avogadros number is a very important relationship to remember. There are 51171021 molecules present in 153g of C6H12O6 glucose. The SI base unit for amount of substance is the mole. It is also possible to get the number of molecules in the given sample by multiplying the number of moles with Avogadro number NA the no. If the substance is molecular the number molecules will be equal to 6022 x 10 23.
 Source: pinterest.com
Source: pinterest.com
We assume you are converting between grams Glucose and mole. How many grams Glucose in 1 mol. Of glucose molecules 001 x 6022 x 10 23 6022 x 10 21 Remember that one mole of any substance contains Avogadro number of particles may be atoms or molecules or ions etc. If the substance is molecular the number molecules will be equal to 6022 x 10 23. This is true of doughnuts pennies grains of sand stars or molecules.
 Source: no.pinterest.com
Source: no.pinterest.com
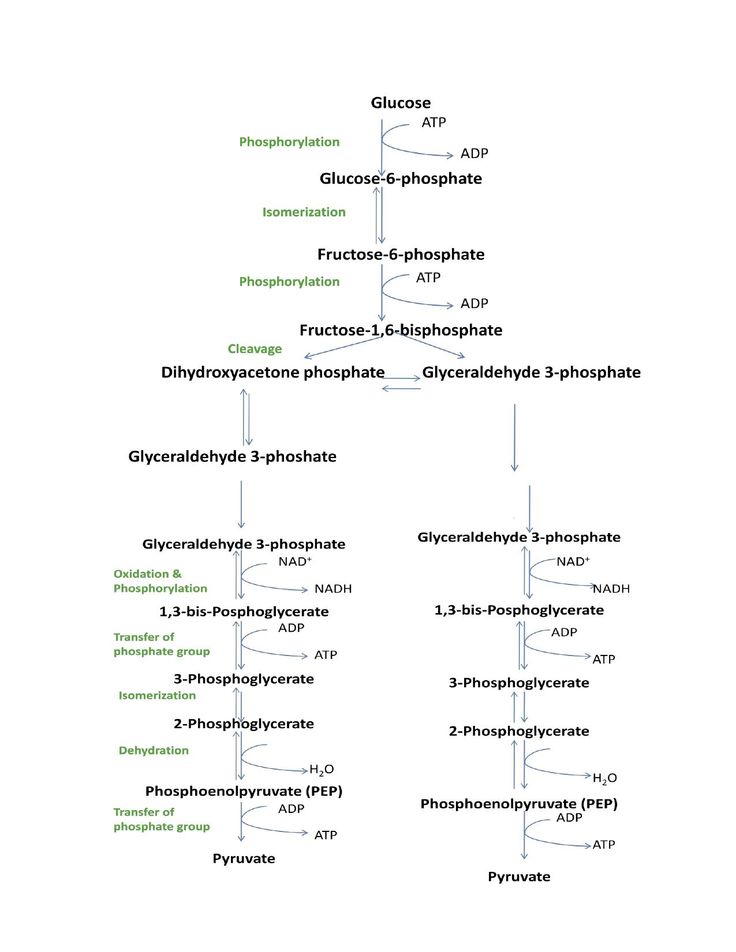
PhD Biochemistry Biophysics Niels Bohr Institutet. Glucose enters the cytoplasm and is broken into 2 molecules of pyruvic acid creating 2 molecules of ATP and 2 molecules of NADH. 1 5 moles A 1 8 m o l e s of B and 2 0 m o l e s of C reacted together according to the reaction A 2 B 4 C A B 2 C 4. 05 moleliter x 1 liter x 6023x1023 moleculesmole 3012x1023 molecules What is the concentration of the 05M glucose solution expressed in. Molecular weight of Glucose or mol The molecular formula for Glucose is C6H12O6.
 Source: pinterest.com
Source: pinterest.com
It is also possible to get the number of molecules in the given sample by multiplying the number of moles with Avogadro number NA the no. We assume you are converting between grams Glucose and mole. Glucose molecular weight. If the substance is molecular the number molecules will be equal to 6022 x 10 23. So the number of molecules present will be.
 Source: cz.pinterest.com
Source: cz.pinterest.com
Glucose enters the cytoplasm and is broken into 2 molecules of pyruvic acid creating 2 molecules of ATP and 2 molecules of NADH. 1 molecule of glucose contains 6 atoms of C 12 atoms of H and 6 atoms of O 1 mole of glucose contains 6 moles of C atoms 12 moles of H atoms and 6 moles of O atoms. The chemical formula of glucose is C12H22O11 C 12 H 22 O 11. PhD Biochemistry Biophysics Niels Bohr Institutet. There are 51171021 molecules present in 153g of C6H12O6 glucose.
 Source: pinterest.com
Source: pinterest.com
Of glucose molecules 001 x 6022 x 10 23 6022 x 10 21 Remember that one mole of any substance contains Avogadro number of particles may be atoms or molecules or ions etc. Next the pyruvic acid enters a mitochondrion where it is completely broken down into 3 molecules of CO2 forming 4 NADH and 1 FADH2 molecules along with 1 ATP molecule. Molar mass of C6H12O6 18015588 gmol. There are 51171021 molecules present in 153g of C6H12O6 glucose. The molar mass of glucose is 6xx1201112xx107946xx1599gmol-1.
 Source: pinterest.com
Source: pinterest.com
This is true of doughnuts pennies grains of sand stars or molecules. There are 00085 or 85103 moles present. Then the number of molecules that could be generated from one glucose molecule could be calculated by dividing the total energy by energy of one mole of ATP ie. This gives an answer of 57 ATP. If the substance is molecular the number molecules will be equal to 6022 x 10 23.
 Source: pinterest.com
Source: pinterest.com
There are 00085 or 85103 moles present. 1 12 60221023 19931023 Note about the mole. So in 1 mol there are Avogadros number of molecules ie. Next the pyruvic acid enters a mitochondrion where it is completely broken down into 3 molecules of CO2 forming 4 NADH and 1 FADH2 molecules along with 1 ATP molecule. Convert grams Glucose to moles or moles Glucose to grams.
 Source: pinterest.com
Source: pinterest.com
So if one mole of glucose contains 6022 1023 molecules of glucose it follows that 400 moles of glucose will contain. Glucose molecular weight. The answer is 18015588. Molar mass of C6H12O6 18015588 gmol. Molecular weight of Glucose or mol The molecular formula for Glucose is C6H12O6.
 Source: pinterest.com
Source: pinterest.com
Hence there are 3011 10 23 molecules in 05moles of glucose. The molar mass of a substance is the mass of one mole of the substance. 400moles Avogadros number 6022 1023molec. 6022xx1023 mol-1 N_A. Molar mass of C6H12O66x12 12x1 6x16 72 12 96 180 g mol-1 Now we can calculate the number of moles of glucose as follows.
This site is an open community for users to do submittion their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site value, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title how many molecules in 1 mole of glucose by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






