Your How many molecules in 1 g of o2 images are available. How many molecules in 1 g of o2 are a topic that is being searched for and liked by netizens today. You can Get the How many molecules in 1 g of o2 files here. Download all royalty-free vectors.
If you’re looking for how many molecules in 1 g of o2 images information linked to the how many molecules in 1 g of o2 interest, you have pay a visit to the right site. Our website always provides you with hints for seeking the maximum quality video and picture content, please kindly surf and locate more informative video content and images that match your interests.
How Many Molecules In 1 G Of O2. 0 2 2 1 0 2 3 x molecules of S O 2 given Now 3 2 g of O 2 6. How many grams of. 3A2 2B C 2D How many moles of D can be formed from 50 mol of A2 and excess B. Its a big number expressed in exponential terms.
 Calculate The Number Of Particles In The 8g Of O2 From toppr.com
Calculate The Number Of Particles In The 8g Of O2 From toppr.com
You can view more details on each measurement unit. 0 2 2 1 0 2 3 x molecules of S O 2 given Now 3 2 g of O 2 6. Thus 1 mole of oxygen molecules means 6022 x 10 23 O 2 molecules or 2 x 6022 x 10 23 O atoms. 1 calculate amount of substance for oxygen gas. Number of O atoms in 16g O2 230111022 60221022 atoms. In other words 1 mole of oxygen would contain molecules.
It can carry a maximum of 4 Oxygen molecules 1 O2 per Heme group.
4 molecules x 2 atoms 8 atoms of oxygen per saturated haemoglobin. You can view more details on each measurement unit. Therefore 256 g of oxygen O2 has 963x 1024 atoms of. B the total number of ions is the same in reactants and products. 682 x 1023 O2 molecules d. How many moles are there in 13 x 1024 atoms of Calcium.
 Source: slideplayer.com
Source: slideplayer.com
2Consider this reaction involving an unknown element X. 0 2 3 1 0 2 3 molecules of O 2 1 g of O 2 3 2 6. The molar mass of Fe is 55845 grams 1 mole Fe 55845 grams Fe. Total molecules in oxygen Mass in gramsMolar mass x N. D the number of atoms of each element is the same in reactants and products.
 Source: toppr.com
Source: toppr.com
Molecular weight of O2 or mol. C the sum of the coefficients of the reactants is equal to the sum of the coefficients of the products. When 3200 g3200 g of XBrXBr reacts 1362 g1362 g of Br2Br2 is produced. The number of molecules in 16 gm of oxygen are 05 1632 moles. You can view more details on each measurement unit.
 Source: youtube.com
Source: youtube.com
Hydrogen peroxide can be prepared in several ways. B the total number of ions is the same in reactants and products. 16g O2 005 mol O2 00560221023 30111022 molecules O2. 292 x 1027 O2 molecules. D the number of atoms of each element is the same in reactants and products.
 Source: slideplayer.com
Source: slideplayer.com
It can carry a maximum of 4 Oxygen molecules 1 O2 per Heme group. Therefore 256 g of oxygen O2 has 963x 1024 atoms of. How many moles of Fe2O3 are there in 100 kg of rust. 737 x 1022 O2 molecules b. And since the chemical formula for water is H 2O 1 mole of water corresponds to 1 mole of oxygen atoms.
 Source: pinterest.com
Source: pinterest.com
The molecular formula for Oxygen is O. NO₂ mO₂ MO₂. Mass of Oxygen 336g. One method is the reaction between hydrogen and oxygen another method is the reaction between water and oxygen. NO₂ 75 g 32 gmol.

The answer is 159994. 737 x 1022 O2 molecules b. MO₂ 75 g. 1 O2 molecule is made up of 2 oxygen atoms. 2 calculate number of molecules.
 Source: pinterest.com
Source: pinterest.com
How many grams of O2 are required to produce 123 1024 molecules of water. NO₂ 0234 mol. NO₂ 75 g 32 gmol. 4 molecules x 2 atoms 8 atoms of oxygen per saturated haemoglobin. In 1 mole of water there are 6022 1023 moleculesthats how the mole was defined.
 Source: in.pinterest.com
Source: in.pinterest.com
MO₂ 75 g. The SI base unit for amount of substance is the mole. Thus 1 mole of oxygen molecules means 6022 x 10 23 O 2 molecules or 2 x 6022 x 10 23 O atoms. MO₂ 75 g. One method is the reaction between hydrogen and oxygen another method is the reaction between water and oxygen.
 Source: es.pinterest.com
Source: es.pinterest.com
1 mol O2 60221023 molecules. If a cylinder of propane contains 100 kg of C3H8 how manygrams of CO2 are frmed when. 347 x 1024 O2 molecules c. It can carry a maximum of 4 Oxygen molecules 1 O2 per Heme group. Now each mole of a substance contains an Avogadros number or of particles.
 Source: toppr.com
Source: toppr.com
The number of molecules in 16 gm of oxygen are 05 1632 moles. How many grams of the excess reactant remain after the reaction is complete. One method is the reaction between hydrogen and oxygen another method is the reaction between water and oxygen. 1 How many molecules of O2 are produced when 421 g of KClO3 decomposes as shown in the reaction. 1255g of Fe 1 x 1 mole 5585 g 225 moles Fe1 x 2 Fe2O3 4 moles Fe 1125 moles Fe2O21 x 1597 g mole x 1788 g Consider the hypothetical reaction.
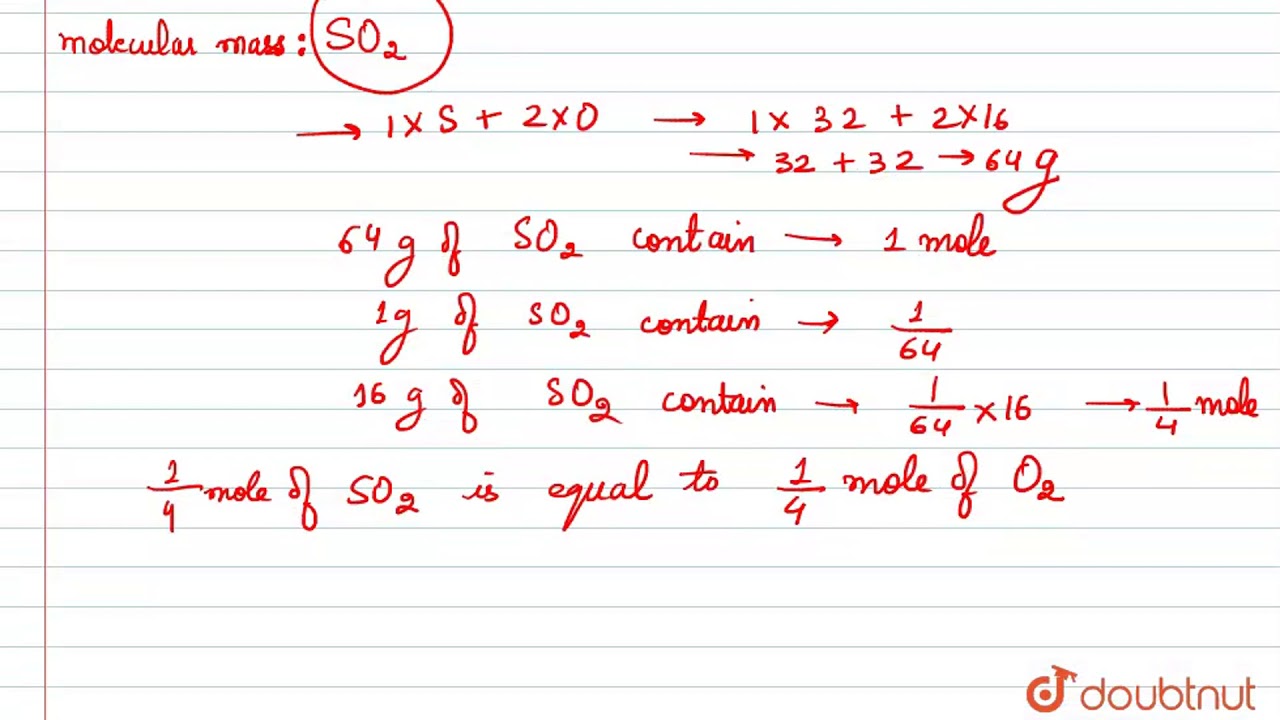 Source: m.youtube.com
Source: m.youtube.com
32008001600 gmol Question 22MultipleChoice Score. 1 How many molecules of O2 are produced when 421 g of KClO3 decomposes as shown in the reaction. There are 14110²³ molecules of oxygen. 1 molecules O2 contains 2 atoms O. 32008001600 gmol Question 22MultipleChoice Score.

One mole of oxygen gas which has the formula O2 has a mass of 32 g and contains 602 X 1023 molecules of oxygen but 1204 X 1023 2 X 602 X 1023 atoms because each molecule of oxygen contains two oxygen atoms. 3A2 2B C 2D How many moles of D can be formed from 50 mol of A2 and excess B. 292 x 1027 O2 molecules. Molar mass of oxygen O2 16 x 2 32gmol. NO₂ 75 g 32 gmol.
 Source: slideplayer.com
Source: slideplayer.com
So 2 Hydrogens and 1 Oxygen 2 1 1 16 18 grams per mole. 347 x 1024 O2 molecules c. NO₂ nO₂ Na. Here we are given with 16 g of oxygen. 2Consider this reaction involving an unknown element X.
 Source: youtube.com
Source: youtube.com
Therefore 256 g of oxygen O2 has 963x 1024 atoms of. How many molecules of CO2 will be formed when 25 molecules ofC3H8 react. Hydrogen peroxide can be prepared in several ways. Use this page to learn how to convert between grams O2 and mole. 0 2 3 1 0 2 3 molecules of O 2.
 Source: br.pinterest.com
Source: br.pinterest.com
1 H2g O2g AP Chem. 1 mol O2 60221023 molecules. Note that rounding errors may occur so always check the results. Therefore 256 g of oxygen O2 has 963x 1024 atoms of. Number of moles 18 g 1008 gmol 2 1600 gmol 18 g 1802 gmol 1 mole.
This site is an open community for users to submit their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site serviceableness, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title how many molecules in 1 g of o2 by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






