Your How many molecules are present in one mole of oxygen images are available in this site. How many molecules are present in one mole of oxygen are a topic that is being searched for and liked by netizens today. You can Find and Download the How many molecules are present in one mole of oxygen files here. Get all royalty-free images.
If you’re looking for how many molecules are present in one mole of oxygen pictures information linked to the how many molecules are present in one mole of oxygen topic, you have visit the right blog. Our website frequently provides you with hints for downloading the maximum quality video and picture content, please kindly hunt and find more enlightening video content and images that match your interests.
How Many Molecules Are Present In One Mole Of Oxygen. Therefore the total number of atoms in 1 mole of SO2 are 60221023moleculesmole3atomsmolecule. How many atoms are in oxygen molecule. One mole of oxygen gas which has the formula O2 has a mass of 32 g and contains 602 X 1023 molecules of oxygen but 1204 X 1023 2 X 602 X 1023 atoms because each molecule of oxygen contains two oxygen atoms. 1 mole elemental oxygen16 grams O1 mole 16 grams.
 One Mole Of Oxygen Gas At Stp Is Equal To Youtube From youtube.com
One Mole Of Oxygen Gas At Stp Is Equal To Youtube From youtube.com
The atomic weight of carbon is 1201 and the atomic weight of chlorine is 3545. Therefore 1 gram molecule of oxygen represents one mole of O2 gas. Oxygen gas is made up of diatomic molecules O2. One mole of oxygen gas which has the formula O 2 has a mass of 32 g and contains 602 X 10 23 molecules of oxygen but 1204 X 10 23 2 X 602 X 10 23 atoms because each molecule of oxygen contains two oxygen atoms. You can view more details on each measurement unit. Moles1 610 23x 1210 22So x 610 231210 22 0201002 moles.
How many atoms are in oxygen molecule.
What is 1g of oxygen. The atomic weight of carbon is 1201 and the atomic weight of chlorine is 3545. Also 1 mole of any substance contains 6022 10 23 number of molecules which is called the Avogadros Number. One mole of oxygen gas which has the formula O 2 has a mass of 32 g and contains 602 X 10 23 molecules of oxygen but 1204 X 10 23 2 X 602 X 10 23 atoms because each molecule of oxygen contains two oxygen atoms. One mole of Water is composed of 1 mole of Oxygen and two moles of Hydrogen. Therefore the total number of atoms in 1 mole of SO2 are 60221023moleculesmole3atomsmolecule.

Therefore 224 litres of oxygen at STP contains 6022 10 23 oxygen molecules. What is 1g of oxygen. To produce two molecules of water H2O it is necessary to combine two molecules of diatomic. Likewise how many molecules are in c9h8o4. The SI base unit for amount of substance is the mole.
 Source: toppr.com
Source: toppr.com
If we total up the gram amounts of each element in the water molecule 15998gmol 21008gmol we get the molar mass of water 18014gmol. 0 2 2 1 0 2 3 8 0 0 2. One mole of O 2 610 23 molecules1210 22 molecules. I read a guys answer here and he said there are 6022 1023 atoms. 1 mole elemental oxygen16 grams O1 mole 16 grams.

The atomic weight of carbon is 1201 and the atomic weight of chlorine is 3545. Also 1 mole of any substance contains 6022 10 23 number of molecules which is called the Avogadros Number. The atomic weight of carbon is 1201 and the atomic weight of chlorine is 3545. The mass of oxygen equal to one mole of oxygen is 15998 grams and the mass of one mole of hydrogen is 1008 g. Therefore 1 gram molecule of oxygen represents one mole of O2 gas.
 Source: youtube.com
Source: youtube.com
There are 12 Carbon atoms 22 Hydrogen atoms and 11 Oxygen atoms in one mol. One mole of oxygen gas which has the formula O2 has a mass of 32 g and contains 602 X 1023 molecules of oxygen but 1204 X 1023 2 X 602 X 1023 atoms because each molecule of oxygen contains two oxygen atoms. Each molecule of O2 is made up of 2 oxygen atoms. One mole of oxygen gas which has the formula O 2 has a mass of 32 g and contains 602 X 10 23 molecules of oxygen but 1204 X 10 23 2 X 602 X 10 23 atoms because each molecule of oxygen contains two oxygen atoms. So take the molecular mass of elemental oxygen - 16 grams per mole.

One atom of sulfur has a mass of 3207 amu. We know that 1 mole of any gas at STP occupies a volume of 224 litres 22400 ml. How many grams are in 1 mole of Fe2O3. 0 2 2 1 0 2 3 8 0 0 ml contains 2 2 4 0 0 6. A mole of O2 molecules has 6023E23 molecules and each molecule is made up is two atoms of oxygen.

How many atoms are in 1g of oxygen. One mole of O 2 610 23 molecules1210 22 molecules. The atomic weight of carbon is 1201 and the atomic weight of chlorine is 3545. So take the molecular mass of elemental oxygen - 16 grams per mole. One mole of oxygen gas which has the formula O2 has a mass of 32 g and contains 602 X 1023 molecules of oxygen but 1204 X 1023 2 X 602 X 1023 atoms because each molecule of oxygen contains two oxygen atoms.
 Source: youtube.com
Source: youtube.com
One mole of O 2 610 23 molecules1210 22 molecules. 1 grams Oxygen is equal to 0062502343837894 mole. For one gram atomic weight of oxygen with atomic weight of 16 grams one mole of oxygen also contains 6022 1023 oxygen atoms. O molecules contains 6022 x 1023 molecules. There are 12 Carbon atoms 22 Hydrogen atoms and 11 Oxygen atoms in one mol.
 Source: youtube.com
Source: youtube.com
What is 1g of oxygen. 1 mole of oxygen contains how many atoms. DOC Week 2 Lab 1. The number of molecules in 16 gm of oxygen are 05 1632 moles. Therefore 1 gram molecule of oxygen represents one mole of O2 gas.

One mole of oxygen gas which has the formula O2 has a mass of 32 g and contains 602 X 1023 molecules of oxygen but 1204 X 1023 2 X 602 X 1023 atoms because each molecule of oxygen contains two oxygen atoms. 0 2 2 1 0 2 3 8 0 0 ml contains 2 2 4 0 0 6. I read a guys answer here and he said there are 6022 1023 atoms. We can clearly see from the formula of SO2 that there is 1 silicon atom and 2 oxygen atoms. Therefore 1 gram molecule of oxygen represents one mole of O2 gas.
 Source: youtube.com
Source: youtube.com
Therefore 224 litres of oxygen at STP contains 6022 10 23 oxygen molecules. Therefore the total number of atoms in 1 mole of SO2 are 60221023moleculesmole3atomsmolecule. How many moles of oxygen are in 1 mole. I read a guys answer here and he said there are 6022 1023 atoms. A mole of O2 molecules has 6023E23 molecules and each molecule is made up is two atoms of oxygen.

DOC Week 2 Lab 1. How many grams are in 1 mole of Fe2O3. How many atoms does. For example one mole of aspirin contains 9 moles of carbon atoms 8 moles of hydrogen atoms and 4 moles of oxygen atoms. Also 1 mole of any substance contains 6022 10 23 number of molecules which is called the Avogadros Number.
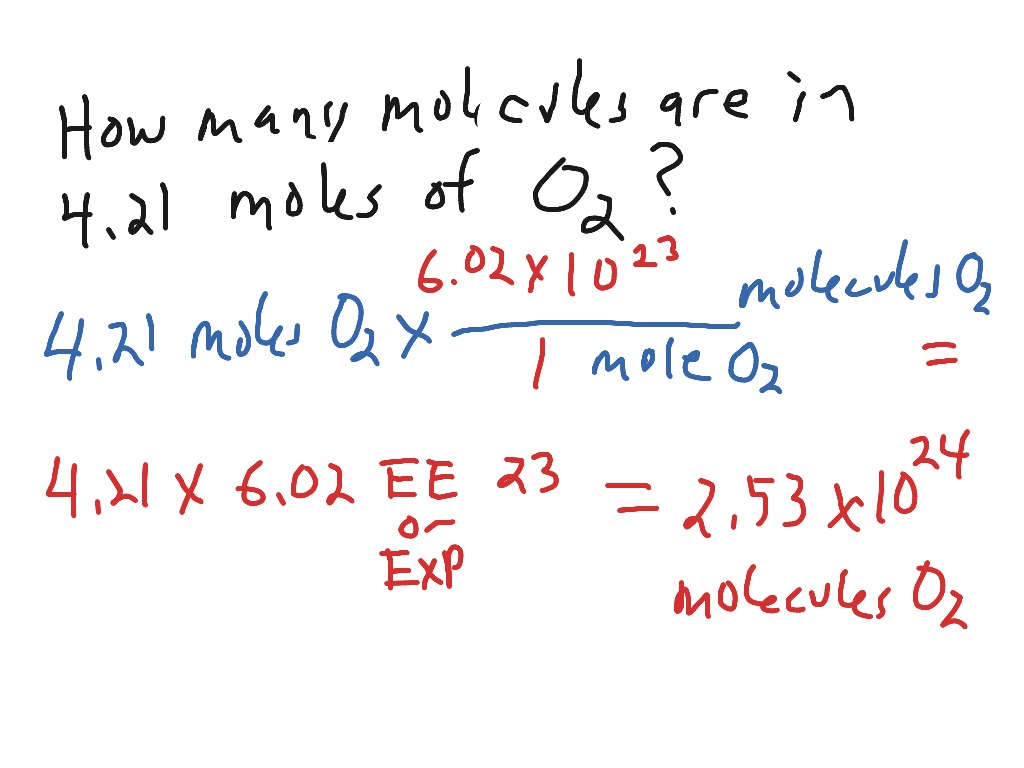 Source: showme.com
Source: showme.com
How many moles of oxygen are in 1 mole. Molecular weight of Oxygen or mol. We are given with 25 moles of SO2 and we need to find the number of atoms in it. How many atoms are in oxygen molecule. Moles1 610 23x 1210 22So x 610 231210 22 0201002 moles.
 Source: youtube.com
Source: youtube.com
One mole of oxygen gas which has the formula O2 has a mass of 32 g and contains 602 X 1023 molecules of oxygen but 1204 X 1023 2 X 602 X 1023 atoms because each molecule of oxygen contains two oxygen atoms. So the molecular weight of CCl4 is 1201 43545 15381 gmol. Moles1 610 23x 1210 22So x 610 231210 22 0201002 moles. Therefore 1 mole of oxygen gas contains 2 moles of oxygen atoms. To produce two molecules of water H2O it is necessary to combine two molecules of diatomic.
 Source: youtube.com
Source: youtube.com
Molecular weight of Oxygen or mol. Now that you know how many moles of aspirin you have in your sample use the fact that one mole of a substance. Likewise how many molecules are in c9h8o4. 1 mole of oxygen contains how many atoms. Therefore 1 mole of oxygen gas is actually 1 mole of O2 molecules.
 Source: slideplayer.com
Source: slideplayer.com
Therefore 1 gram molecule of oxygen represents one mole of O2 gas. 1 mole contains 6022 x 1023 entities Avogadros number Concept 1. Therefore 1 mole of oxygen gas contains 2 moles of oxygen atoms. Thus the number of molecules in 16 gm or 05 mole of oxygen gas are 05 x 6023 x 1023 3012 x 1023 molecules. There are 12 Carbon atoms 22 Hydrogen atoms and 11 Oxygen atoms in one mol.
This site is an open community for users to submit their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site serviceableness, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title how many molecules are present in one mole of oxygen by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






