Your How many molecules are present in a mole of glucose c6h12o6 images are available in this site. How many molecules are present in a mole of glucose c6h12o6 are a topic that is being searched for and liked by netizens today. You can Download the How many molecules are present in a mole of glucose c6h12o6 files here. Find and Download all free photos.
If you’re searching for how many molecules are present in a mole of glucose c6h12o6 images information related to the how many molecules are present in a mole of glucose c6h12o6 keyword, you have visit the right site. Our site always provides you with hints for seeking the highest quality video and image content, please kindly surf and find more enlightening video content and images that match your interests.
How Many Molecules Are Present In A Mole Of Glucose C6h12o6. Putting values in above equation we get. Mass of 1 mole of glucose C6H12O6 6 1201 12 101 6 1600 g 18018 g using atomic weight data to 2 decimals 1 mole of carbon atoms weighs 1201 g and there are 6 moles of C atoms in 1 mole of glucose so the. 1 mole of a compound contains number of molecules. Molecular weight of C6H12O6 or mol.

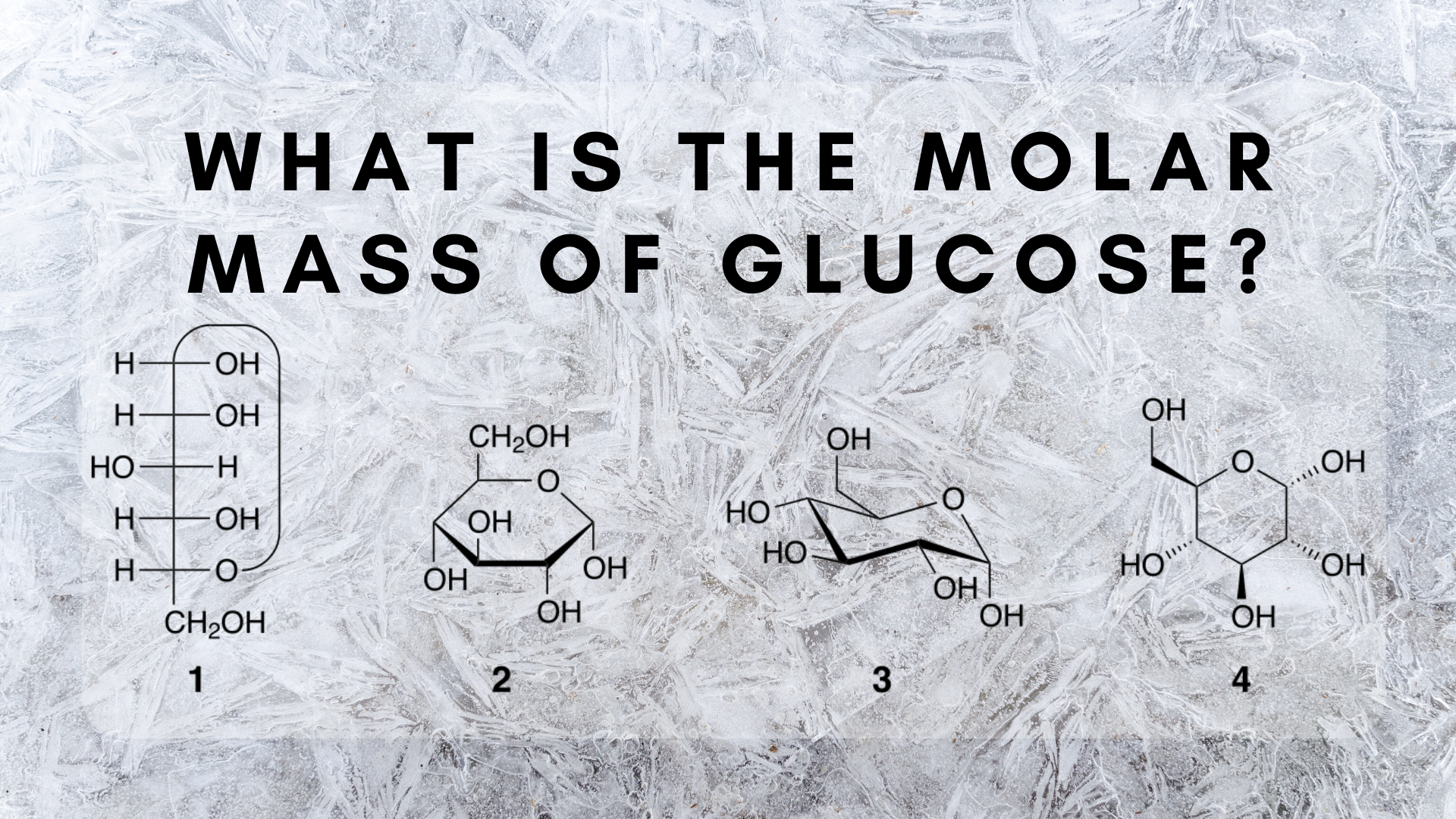
You can view more details on each measurement unit. According to the Avogadros number one mole of a substance contain 6022x 10 23 molecules. Hence the number of molecules present in given amount of glucose are. So atoms in one mole of glucose is. How many moles is this. From the molecular formula C 6 H 12 O 6 one can find there are 6 carbon atoms 12 hydrogen atoms and 6 oxygen atoms in one molecule of glucose.
Moles of oxygen atoms 6 moles of glucose.
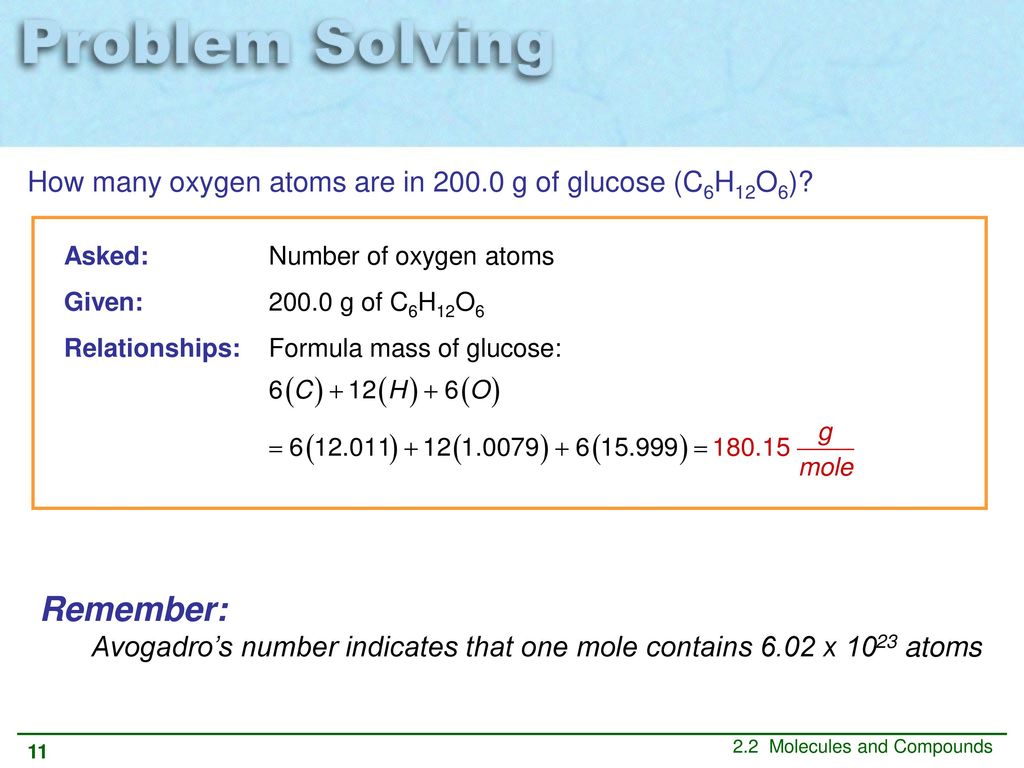
Molar mass of H 1 g mol -1. From the molecular formula C6H12O6 one can find there are 6 carbon atoms 12 hydrogen atoms and 6 oxygen atoms in one molecule of glucose. So 519 moles of a compound will contain number of molecules. 1201076 10079412 1599946. There are 00085 or 85103 moles present. Molar mass of glucose 18016 gmol.
 Source: brainly.in
Source: brainly.in
How many moles of carbon are in C6H12O6. With these moles and Avogadros number we find the total number of glucose molecules present. 96 g oxygen is a molar quantity of 96 g 16 g mol1 6 mol with respect to oxygen atoms. Molar mass of H 1 g mol -1. 132 x 12 x 602 x1023 954 x 1024 hydrogen atoms in 132 moles of glucose.
 Source: chegg.com
Source: chegg.com
How many molecules are in 6g of glucose. 1 mole of a compound contains number of molecules. The molar mass of glucose can be calculated from the molar masses of individual atoms present in it. There are 12 hydrogen atoms in 1 molecule of glucose there are Avogadroa number 602 x 1023 atoms in one mole of any substance. One mole of glucose 6022x 10 23 molecules three moles of glucose 36022x 10 23 molecules In a molecule of glucose there are 24 atoms.
 Source: slideplayer.com
Source: slideplayer.com
Molar mass of C 12 g mol -1. How many moles is this. 132 x 12 x 602 x1023 954 x 1024 hydrogen atoms in 132 moles of glucose. There are 51171021 molecules present in 153g of C6H12O6 glucose. Moles of oxygen atoms 6 moles of glucose.

The molecular formula of Glucose is C₆H₁₂O₆. Mass of 1 mole of glucose C6H12O6 6 1201 12 101 6 1600 g 18018 g using atomic weight data to 2 decimals 1 mole of carbon atoms weighs 1201 g and there are 6 moles of C atoms in 1 mole of glucose so the. One mole of glucose 246022x 10 23 atoms three moles of glucose 3246022x 10. Mass of glucose 936 g. One mole of glucose 6022x 10 23 molecules three moles of glucose 36022x 10 23 molecules In a molecule of glucose there are 24 atoms.
 Source: sciencetrends.com
Source: sciencetrends.com
How many molecules of sugar C6H12O6 are in 6 g of sugar. What is the number of oxygen atoms present in 5 mole of glucose. Also question is how many molecules are present in a mole of glucose c6h12o6. A person drinks 150 103 g of water H2O per day. We assume you are converting between grams C6H12O6 and mole.
 Source: clutchprep.com
Source: clutchprep.com
132 x 12 x 602 x1023 954 x 1024 hydrogen atoms in 132 moles of glucose. So 519 moles of a compound will contain number of molecules. Now according to the mole concept. Eqrm glucose molecules 000833 mol times dfrac6022 times 1023mol. We assume you are converting between grams C6H12O6 and mole.
 Source: chegg.com
Source: chegg.com
Now according to the mole concept. One mole of glucose 6022x 10 23 molecules three moles of glucose 36022x 10 23 molecules In a molecule of glucose there are 24 atoms. One mole of glucose 246022x 10 23 atoms three moles of glucose 3246022x 10. The molar mass of glucose can be calculated from the molar masses of individual atoms present in it. To be completely accurate please note that the SI definition of a mole the globally standard unit for the amount of something is 602214076 x 1023 elementary units of a substance.
 Source: slideplayer.com
Source: slideplayer.com
Glucose is C6H12O6 and Avogadros Number named for Amadeo Carlo Avogadro 1776 1856 tells us that 1 mole contains 6022 x 1023 molecules. So 519 moles of a compound will contain number of molecules. How many moles of carbon are in C6H12O6. Molecular weight of C6H12O6 or mol. 96 g oxygen is a molar quantity of 96 g 16 g mol1 6 mol with respect to oxygen atoms.

Also question is how many molecules are present in a mole of glucose c6h12o6. Hence the number of molecules present in given amount of glucose are. There are 51171021 molecules present in 153g of C6H12O6 glucose. Mass of 1 mole of glucose C6H12O6 6 1201 12 101 6 1600 g 18018 g using atomic weight data to 2 decimals 1 mole of carbon atoms weighs 1201 g and there are 6 moles of C atoms in 1 mole of glucose so the. Convert grams C6H12O6 to moles or moles C6H12O6 to grams.
 Source: clutchprep.com
Source: clutchprep.com
The answer is 18015588. Moles of oxygen atoms 6 moles of glucose. You can view more details on each measurement unit. With these moles and Avogadros number we find the total number of glucose molecules present. Mass of 1 mole of glucose C6H12O6 6 1201 12 101 6 1600 g 18018 g using atomic weight data to 2 decimals 1 mole of carbon atoms weighs 1201 g and there are 6 moles of C atoms in 1 mole of glucose so the.
 Source: youtube.com
Source: youtube.com
So atoms in one mole of glucose is. Glucose is C6H12O6 and Avogadros Number named for Amadeo Carlo Avogadro 1776 1856 tells us that 1 mole contains 6022 x 1023 molecules. There are 12 hydrogen atoms in 1 molecule of glucose there are Avogadroa number 602 x 1023 atoms in one mole of any substance. How many molecules are present in a mole of glucose C6H12O6. This means one mol of glucose contains 6 mol of oxygen.
 Source: youtube.com
Source: youtube.com
Molar mass of glucose 18016 gmol. Eqrm glucose molecules 000833 mol times dfrac6022 times 1023mol. How many moles of carbon are in C6H12O6. A person drinks 150 103 g of water H2O per day. You can view more details on each measurement unit.
 Source: youtube.com
Source: youtube.com
Molar mass of H 1 g mol -1. Calculation of molar mass of glucose C6H12O6 The molar mass of glucose can be calculated from the molar masses of individual atoms present in it. Now according to the mole concept. Molar mass of C6H12O6 18015588 gmol. There are 12 hydrogen atoms in 1 molecule of glucose there are Avogadroa number 602 x 1023 atoms in one mole of any substance.
 Source: youtube.com
Source: youtube.com
How many hydrogen atoms are there in 250 mol of C6H12O6. How many moles of carbon are in C6H12O6. Convert grams C6H12O6 to moles or moles C6H12O6 to grams. There are 00085 or 85103 moles present. Each glucose molecule is composed of C6H12O6.
 Source: toppr.com
Source: toppr.com
One mole is 6021023 molecules. The molar mass of glucose can be calculated from the molar masses of individual atoms present in it. How many molecules are in 6g of glucose. This means one mol of glucose contains 6 mol of oxygen. So one mole of sugar glucose - C6H12O6 is equal to 6022 x 1023 molecules of sugar.
This site is an open community for users to do sharing their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site adventageous, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title how many molecules are present in a mole of glucose c6h12o6 by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






