Your How many molecules are present in 1 mole of water images are ready in this website. How many molecules are present in 1 mole of water are a topic that is being searched for and liked by netizens now. You can Get the How many molecules are present in 1 mole of water files here. Download all free photos and vectors.
If you’re searching for how many molecules are present in 1 mole of water pictures information linked to the how many molecules are present in 1 mole of water keyword, you have visit the right site. Our site always provides you with suggestions for seeking the maximum quality video and picture content, please kindly search and locate more enlightening video articles and images that fit your interests.
How Many Molecules Are Present In 1 Mole Of Water. So 224 liters of water has 6022 X 1023 molecules 1 liter of water has 6022 X 1023224 molecules. 268 X 1021 molecules. The answer is 1801528. The number of moles of water will be.
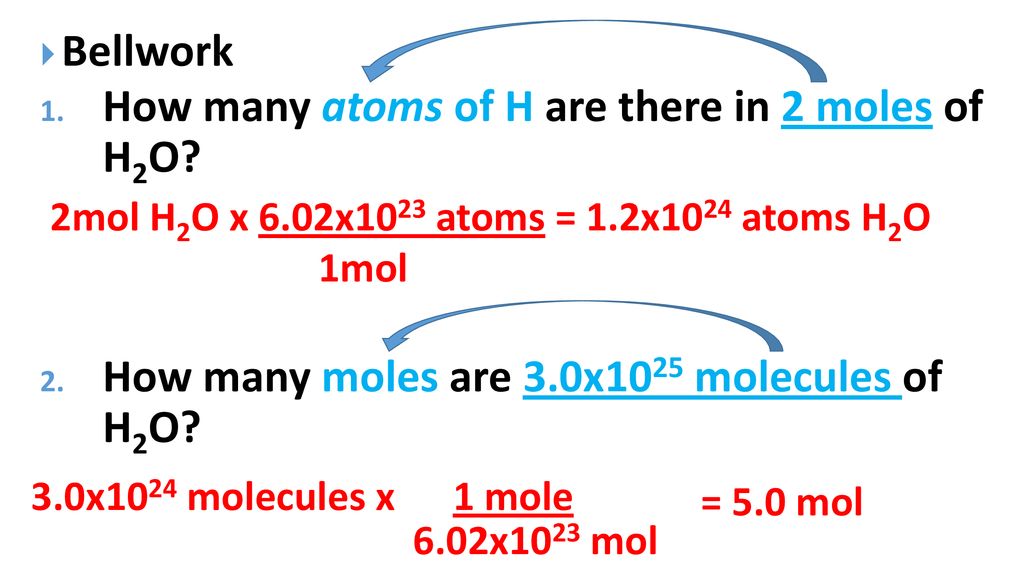 How Many Atoms Of H Are There In 2 Moles Of H2o Ppt Download From slideplayer.com
How Many Atoms Of H Are There In 2 Moles Of H2o Ppt Download From slideplayer.com
Since we only have 0001 grams that means that we have NA 000118 3351019 molecules of water. If we take atomic substance then the number of atoms in one mole is equal to Avogadro number. 1008 gramsmole Hydrogen 1 mole6022x1023 atoms 167 x 10-24 grams. How many moles are in 435 grams of water. We can thus say that for 1 ml of water the number of moles is dfrac118. So a mole of water is 602 x 1023 molecules of water which works out to be about 18 grams or 18 mL.
100ml of water has 268 X 1021 molecules.
How many atoms are contained in 23 g. But each molecule of water contains 2 H and 1 O atom 3 atoms so there are approximately 18 x 1024 atoms in a mole of water. Molecular weight of Water or mol. View solution 2 g of hydrogen will have same number of molecules as is present in. Or 18 g of water contains 6022 x 1023 water molecules. We can thus say that for 1 ml of water the number of moles is dfrac118.

100ml of water has 268 X 1021 molecules. These numbers are either in atomic mass units amu or in grams per mole of atoms. 1 molecules is equal to 1660538863127E-24 mole. Because the mole contains so many units theyre most often used in chemistry is a way of measuring really really small things like atoms or molecules. 1 mole of water molecule has 6022 X 1023 molecules 1 mole is equivalent to 224litres.
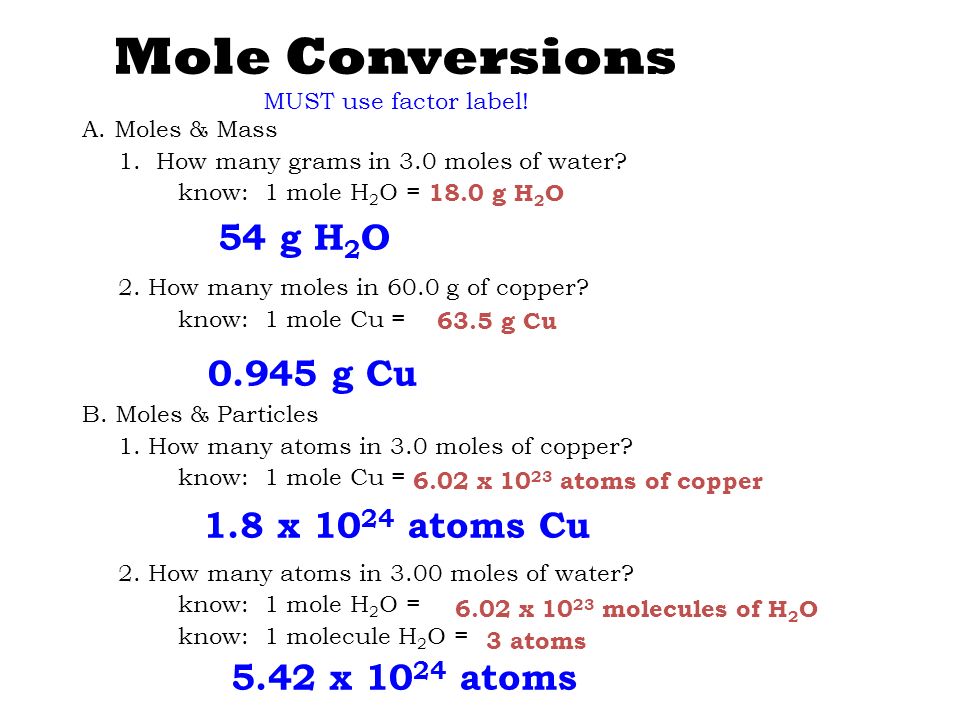 Source: slideplayer.com
Source: slideplayer.com
1 mole of water molecule has 6022 X 1023 molecules 1 mole is equivalent to 224litres. Since we only have 0001 grams that means that we have NA 000118 3351019 molecules of water. But each molecule of water contains 2 H and 1 O atom 3 atoms so there are approximately 18 x. The number of moles of water will be. How many moles are contained in 15055 x 10 molecules of a compound.

So now that you know how many molecules are needed to have 1 mole of water use Avogadros number as a conversion factor to calculate the number of molecules present in your sample. Avogadros number tells us there are 6022 x 10 23 molecules of water per mole of water. Soobee72pl and 13 more users found this answer. So a mole of water is 602 x 1023 molecules of water which works out to be about 18 grams or 18 mL. How many moles are contained in 15055 x 10 molecules of a compound.
 Source: slideplayer.com
Source: slideplayer.com
1 molecules is equal to 1660538863127E-24 mole. If u like plzmark it brainliest-. One mole of water contains 602 x 1023 molecules of water. Molecular weight of H 2 O 1818 g water 18 ml waterBecause density of water 1 18 ml of H 2 O contain60210 23 molecules of H 2 O 1ml of H 2 O contain 1860210 23 3310 22. 100ml of water has 268 X 1021 molecules.
 Source: pinterest.com
Source: pinterest.com
If we have one mole of water then we know that it will have a mass of 2 grams for 2 moles of H atoms 16 grams for one mole O atom 18 grams. If we take atomic substance then the number of atoms in one mole is equal to Avogadro number. Of molecules in 1ml water no. 18 g of water contains 1 mol of water molecules. The molecular weight of water is 18 gmmol.
 Source: slideplayer.com
Source: slideplayer.com
So 224 liters of water has 6022 X 1023 molecules 1 liter of water has 6022 X 1023224 molecules. View solution The number of water molecules present in a drop of water weighing 0. Of moles x avogadros no. 25 mol 602 1023 1 mol 15 1024 molecules. If u like plzmark it brainliest-.

Here given mass 1ml 1gm. One mole of water contains 602 x 1023 MOLECULES of water. These numbers are either in atomic mass units amu or in grams per mole of atoms. Ar 4s 2 3d 7. How many atoms are contained in 23 g.
 Source: in.pinterest.com
Source: in.pinterest.com
How many moles are contained in 48 g ofmethane CH C 12 ug H-1 u 22. From the periodic table we see the atomic weight of hydrogen is 10079 and the atomic weight of oxygen is 159994. The number of moles of water will be. The molecular formula for Water is H2O. Because the mole contains so many units theyre most often used in chemistry is a way of measuring really really small things like atoms or molecules.
 Source: socratic.org
Source: socratic.org
These numbers are either in atomic mass units amu or in grams per mole of atoms. The number of moles of water will be. Soobee72pl and 13 more users found this answer. How many moles are contained in 48 g ofmethane CH C 12 ug H-1 u 22. But each molecule of water contains 2 H and 1 O atom 3 atoms so there are approximately 18 x 1024 atoms in a mole of water.
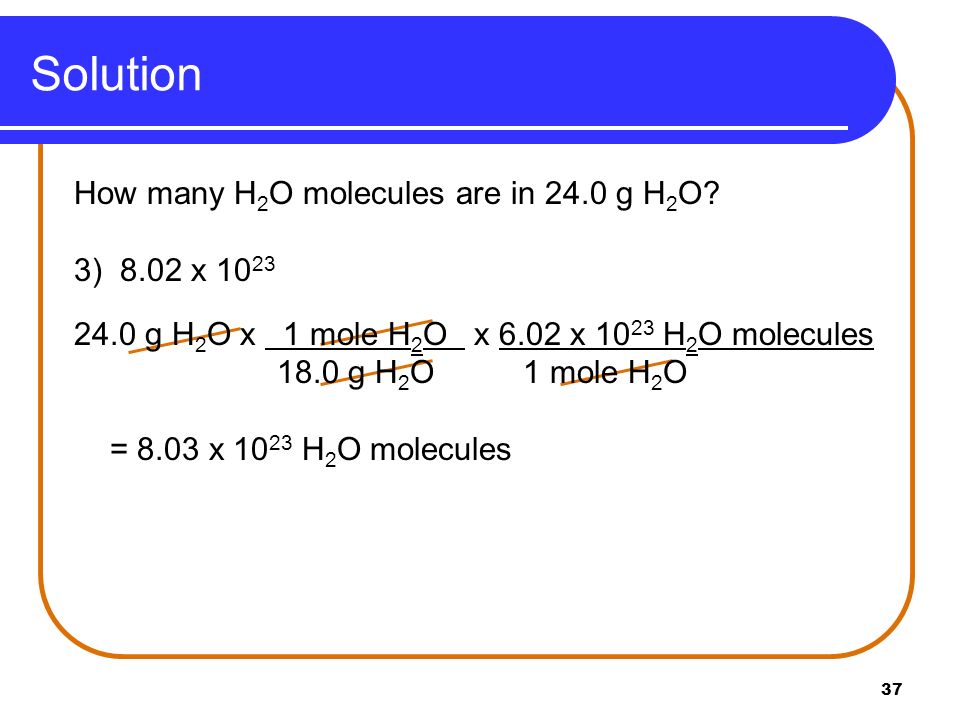 Source: slideplayer.com
Source: slideplayer.com
Here given mass 1ml 1gm. You can calculate the number of molecules from moles by using Avogadros constant 602 1023 moleculesmol. Soobee72pl and 13 more users found this answer. Of molecules of water in 1ml of water 33 x 1022. You can view more details on each measurement unit.
 Source: youtube.com
Source: youtube.com
25 mol 602 1023 1 mol 15 1024 molecules. 100ml of water has 268 X 1021 molecules. From the periodic table we see the atomic weight of hydrogen is 10079 and the atomic weight of oxygen is 159994. 250moles H2O 6022 1023molec. One mole of water contains 602 x 1023 MOLECULES of water.

But each molecule of water contains 2 H and 1 O atom 3 atoms so there are approximately 18 x 1024 atoms in a mole of water. 100ml of water has 268 X 1021 molecules. So 224 liters of water has 6022 X 1023 molecules 1 liter of water has 6022 X 1023224 molecules. Molecular weight of H 2 O 1818 g water 18 ml waterBecause density of water 1 18 ml of H 2 O contain60210 23 molecules of H 2 O 1ml of H 2 O contain 1860210 23 3310 22. H2O 1mole H2O a a 1.
 Source: in.pinterest.com
Source: in.pinterest.com
18 g of water contains 1 mol of water molecules. The number of moles of water will be. Avogadros number tells us there are 6022 x 10 23 molecules of water per mole of water. Put another way there are 167 sextillion water molecules in a water drop. View solution 2 g of hydrogen will have same number of molecules as is present in.
 Source: clutchprep.com
Source: clutchprep.com
0 1 8. The SI base unit for amount of substance is the mole. Of moles x avogadros no. In 1 mole of any substance the number of molecules present is N_A. 1 mol water 6023 1023 molecules.
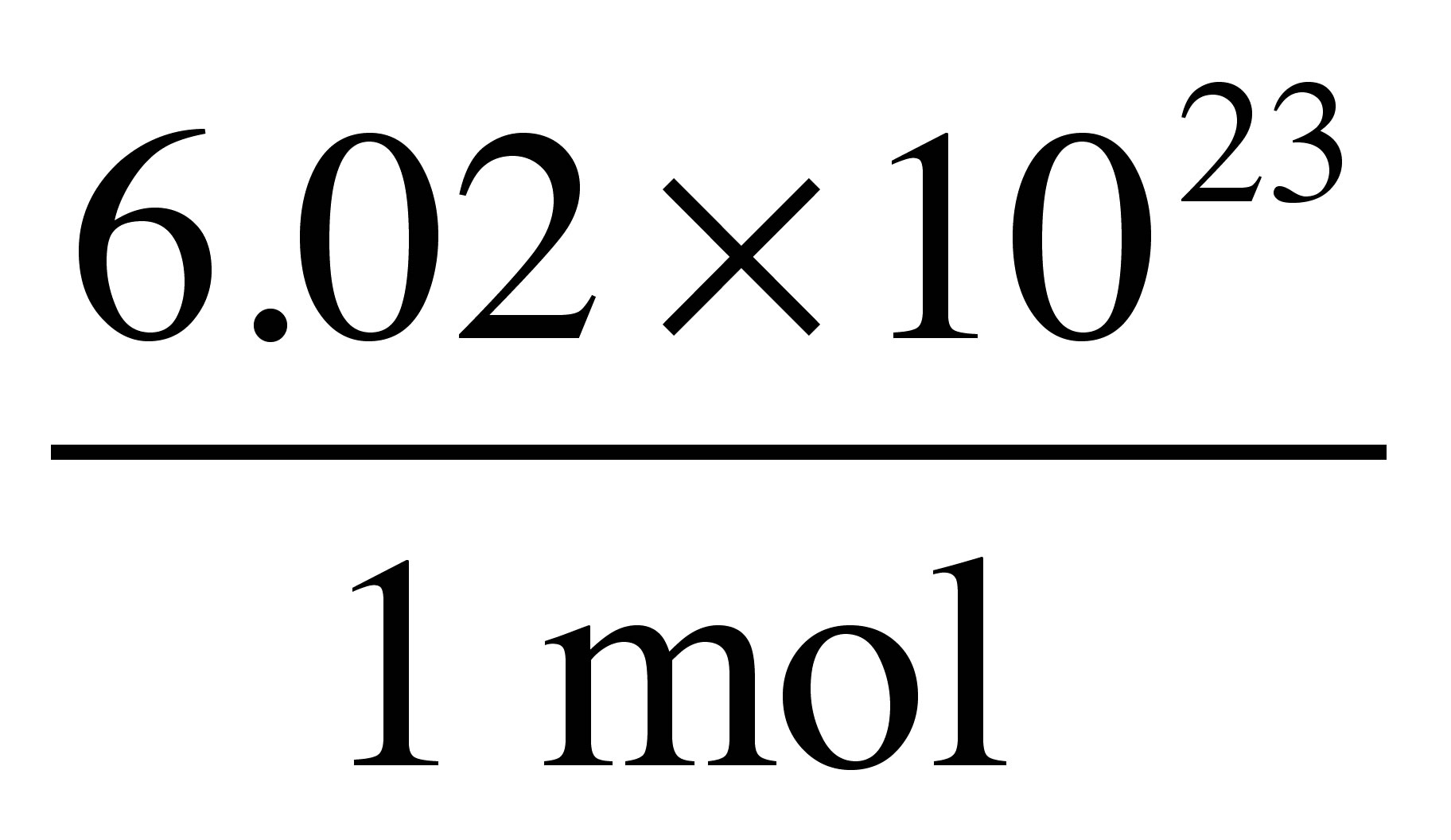 Source: socratic.org
Source: socratic.org
Since one mole is defined as 6022 x 1023 and there are two hydrogen per molecule that would mean that there are exactly 12044 x 1023 molecules of hydrogen in one mole of water. This means that 1 MOLE of hydrogen atoms will weigh 1008 grams. -We all know that. Ar 4s 2 3d 7. View solution 2 g of hydrogen will have same number of molecules as is present in.
This site is an open community for users to do submittion their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site value, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title how many molecules are present in 1 mole of water by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






