Your How many molecules are in one mole of nacl images are ready in this website. How many molecules are in one mole of nacl are a topic that is being searched for and liked by netizens today. You can Find and Download the How many molecules are in one mole of nacl files here. Find and Download all royalty-free photos and vectors.
If you’re looking for how many molecules are in one mole of nacl images information linked to the how many molecules are in one mole of nacl interest, you have pay a visit to the ideal site. Our website frequently provides you with suggestions for downloading the highest quality video and image content, please kindly search and find more enlightening video articles and graphics that fit your interests.
How Many Molecules Are In One Mole Of Nacl. Quick conversion chart of grams NaCl to mol. The SI base unit for amount of substance is the mole. Moles 50585 0855 moles 3sf. Now you can do some weight percentage problems like.
 7 4 The Mole A Counting Term States A Specific Number Of Items The Terms Dozen Case Gross And Ream Are Used To Count The Number Of Items Present Ppt Download From slideplayer.com
7 4 The Mole A Counting Term States A Specific Number Of Items The Terms Dozen Case Gross And Ream Are Used To Count The Number Of Items Present Ppt Download From slideplayer.com
Since there are 012 moles NaCl apparently there are 012 moles of each of the elements. Each molecule of NaCl has two atoms and so number of atoms in one mole NaCl 2602210²³ 120441024 Each molecule of NaCl has two atoms and so number of atoms in one mole NaCl 2602210²³ 120441024. One mole is equal to 6022141791023 atoms or other elementary units such as molecules. 1 Answer Junaid Mirza Apr 20 2018 0342 mol Explanation. Here is the general way of solving this kind of problems. What is the equations for how many molecules are in 3 moles of NaCl.
How many moles of NaCl are present in 200 g of NaCl.
Finding molar mass starts with units of grams per mole gmol. 1 mol of NaCl 602 1023 formula units. 5844 gmol is the molecular weight of NaCl. 10 grams NaCl to mol 017111 mol. Click to see full answer. Na has an atomic mass of 23 and Cl is 355.
 Source: pinterest.com
Source: pinterest.com
Now you can do some weight percentage problems like. Isnt avagadros number fun. That would be 05 moles of Cl 2. If I take NaCl a mole of molecules then there are 2 moles of Na and 2 moles of Cl₂ molecules in both sides or 1 mole of Na and 0s moles of Cl₂ molecules present. Quick conversion chart of grams NaCl to mol.
 Source: pinterest.com
Source: pinterest.com
That would be 05 moles of Cl 2. We do this by looking up the relative atomic masses of Na and Cl in the periodic table. Thus 1 mole of NaCl will contain 6023 x 1023 atoms each of Na and Cl or a total of 12046 x 1023 atoms. NaCl is an ionic compound so there are no molecules only formula units. Quick conversion chart of grams NaCl to mol.
 Source: slideplayer.com
Source: slideplayer.com
One unit of sodium chloride is called a functional unit. Chemistry The Mole Concept The Mole. How many moles of NaCl are in 585 g of NaCl. 30 grams NaCl to mol 051332 mol. 1 mole is equal to 1 moles NaCl or 5844277 grams.
 Source: youtube.com
Source: youtube.com
When calculating molecular weight of a chemical compound it tells us how many grams are in one mole of that substance. 124 1024 formula units. Na has an atomic mass of 23 and Cl is 355. Formula units NaCl 120 g NaCl 1 mol NaCl 5844 g NaCl 602 1023 formula units 1 mol NaCl. That would be 05 moles of Cl 2.
 Source: pinterest.com
Source: pinterest.com
Furthermore how many particles are there in CaCl2. The formula weight is simply the weight in atomic mass units of all the atoms in a given formula. 133K views View upvotes Related Answer Muhammad Attaullah BS Industrial Chemistry from GC University Faisalabad. Now you can use these numbers in the equations. 1 mole is equal to 1 moles NaOH or 3999711 grams.
 Source: thefactfactor.com
Source: thefactfactor.com
On average a grain of table salt Sodium Chloride NaCl has a mass of about 100 g one ten-thousandth of a gram. One unit of sodium chloride is called a functional unit. The SI base unit for amount of substance is the mole. As given in the Periodic Table of Elements these are equal to M M Na 2298977 g mol1 M MCl 354527 g mol1 You thus have M M NaCl 2298977 g mol1 354527 g mol1 M M NaCl 5844247 g mol1 This means that the mass of one mole of sodium chloride is equal to. 1 mole of NaCl contains 2 moles of ions 1 mole of sodium ions and 1 mole of chloride ions according to the following expression- NaCl Na Cl- Hence 25 mole of NaCl will contain 5 moles of total ions.
 Source: es.pinterest.com
Source: es.pinterest.com
The SI base unit for amount of substance is the mole. That would be 05 moles of Cl 2. But since each individual molecule consists of 2 atoms of nitrogen the number of moles of nitrogen atoms will be twice that of nitrogen gas molecules. A common request on this site is to convert grams to moles. The mole abbreviated mol is an SI unit which measures the number of particles in a specific substance.
 Source: chemiris.labs.brocku.ca
Source: chemiris.labs.brocku.ca
Click to see full answer. If I take NaCl a mole of molecules then there are 2 moles of Na and 2 moles of Cl₂ molecules in both sides or 1 mole of Na and 0s moles of Cl₂ molecules present. How many moles of NaCl are in 585 g of NaCl. Now you can do some weight percentage problems like. 10 grams NaCl to mol 017111 mol.
 Source: pinterest.com
Source: pinterest.com
435 1345 Views. How many moles of NaCl are present in 200 g of NaCl. Besides one mole of Sodium Chloride NaCl has a mass of approximately 585 grams. 435 1345 Views. We do this by looking up the relative atomic masses of Na and Cl in the periodic table.
 Source: pinterest.com
Source: pinterest.com
Moles 50585 0855 moles 3sf. 30 grams NaCl to mol 051332 mol. Chemistry The Mole Concept The Mole. Jun 30 2017. Click to see full answer.
 Source: pinterest.com
Source: pinterest.com
Jun 30 2017. This tells us that there are 0427 moles of NaCl in the solution. Forums Other Sciences Chemistry. If you had 20g of NaCl what percent of the weight is due to the presence of sodium. Furthermore how many particles are there in CaCl2.
 Source: pinterest.com
Source: pinterest.com
Molar mass of NaCl 584 gmol Number of moles in. Now you can do some weight percentage problems like. 30 grams NaCl to mol 051332 mol. 1 Answer Junaid Mirza Apr 20 2018 0342 mol Explanation. Forums Other Sciences Chemistry.
 Source: socratic.org
Source: socratic.org
Na has an atomic mass of 23 and Cl is 355. Forums Other Sciences Chemistry. One mole is equal to 6022141791023 atoms or other elementary units such as molecules. Quick conversion chart of grams NaCl to mol. The mole abbreviated mol is an SI unit which measures the number of particles in a specific substance.
 Source: slideplayer.com
Source: slideplayer.com
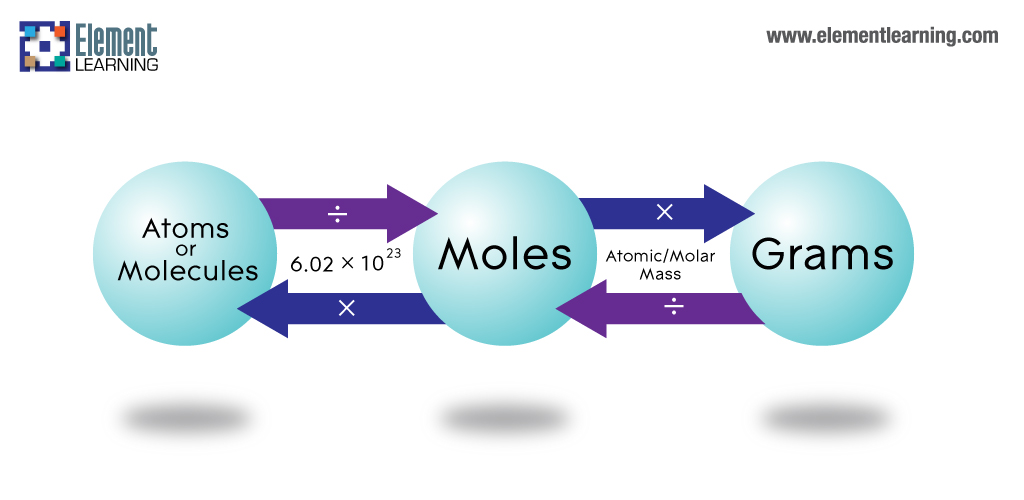
Chemistry The Mole Concept The Mole. Here is the general way of solving this kind of problems. Now you can do some weight percentage problems like. The number of molecules in a substance can be attained through Avogadros number 6022 x 10 23 molecules per mole of compound. Na has an atomic mass of 23 and Cl is 355.
 Source: slideplayer.com
Source: slideplayer.com
100 grams NaCl to mol 171108 mol. Each molecule of NaCl has two atoms and so number of atoms in one mole NaCl 2602210²³ 120441024 Each molecule of NaCl has two atoms and so number of atoms in one mole NaCl 2602210²³ 120441024. 1 grams NaCl to mol 001711 mol. To calculate the number of molecules in a given number of mole we can simply multiply by Avogadros number which is equal to 6022xx1023. Forums Other Sciences Chemistry.
This site is an open community for users to share their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site helpful, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title how many molecules are in one mole of nacl by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






