Your How many molecules are in one mole of carbon dioxide co2 images are ready. How many molecules are in one mole of carbon dioxide co2 are a topic that is being searched for and liked by netizens now. You can Get the How many molecules are in one mole of carbon dioxide co2 files here. Get all free vectors.
If you’re searching for how many molecules are in one mole of carbon dioxide co2 images information connected with to the how many molecules are in one mole of carbon dioxide co2 keyword, you have visit the right blog. Our website frequently provides you with hints for seeing the highest quality video and picture content, please kindly surf and find more informative video articles and graphics that fit your interests.
How Many Molecules Are In One Mole Of Carbon Dioxide Co2. The mass of one mole of CO2 is 4401 grams. Use this page to learn how to convert between grams Carbon Dioxide and mole. Of oxygen atoms in 88 g of CO2 contains is 2260221023 atoms. Understanding the last step is critical.

Of oxygen atoms in 88 g of CO2 contains is 2260221023 atoms. Moreover how many atoms are there in 1 mole of co2. Carbon has a molar mass of 120107 gmol and oxygen has a molar mass of 159994 gmol. Use this page to learn how to convert between grams Carbon Dioxide and mole. Lets consider the carbon dioxide molecule. Jan 17 2020 Avogadros quantity reveals us that there are 6022 x 1023 molecules of CO2 in 1 mole of the gasoline.
How many molecules are in 2 moles of CO2.
1 6022102360221023 molecules of CO2 are present. 730 moles carbon dioxide 6022 X 10231 mole CO2 440 X 1024 molecules of carbon dioxide. Ka735 x 1023 mass CO2 4401 b. 1 grams Carbon Dioxide is equal to 0022722366761722 mole. One mole of carbon contains 6022 1023 atoms of carbon. The SI base unit for amount of substance is the mole.

Chemistry questions and answers. Lets consider the carbon dioxide molecule. Carbon has a molar mass of 120107 gmol and oxygen has a molar mass of 159994 gmol. So the molar mass of carbon dioxide is 4401 gmol. Jan 17 2020 Avogadros quantity reveals us that there are 6022 x 1023 molecules of CO2 in 1 mole of the gasoline.

You can view more details on each measurement unit. Carbon has a molar mass of 120107 gmol and oxygen has a molar mass of 159994 gmol. How large is a carbon dioxide molecule. We know it has the formula CO2 and this tells us that. Therefore 22 g of CO2 will contain 3011 x 1023 molecules.
 Source: sites.google.com
Source: sites.google.com
And we take the product of the quotient. Ka735 x 1023 mass CO2 4401 b. Now we know that one molecule of CO contains one atom of oxygen. Understanding the last step is critical. Now in mol of CO2 there are 6022x10 23.

And we take the product of the quotient. How many molecules are in 2 moles of CO2. 250L x 1 mol224 L 0112 moles CO 2. Use this page to learn how to convert between grams Carbon Dioxide and mole. How do you calculate oxygen atoms in CO2.

730 moles carbon dioxide 6022 X 10231 mole CO2 440 X 1024 molecules of carbon dioxide. How many molecules of carbon dioxide CO comprise 122 moles. 120107159994 2 4401 gmol. Where N A Avocado number 6022 1023 mol1. How many molecules are in 2 moles of CO2.
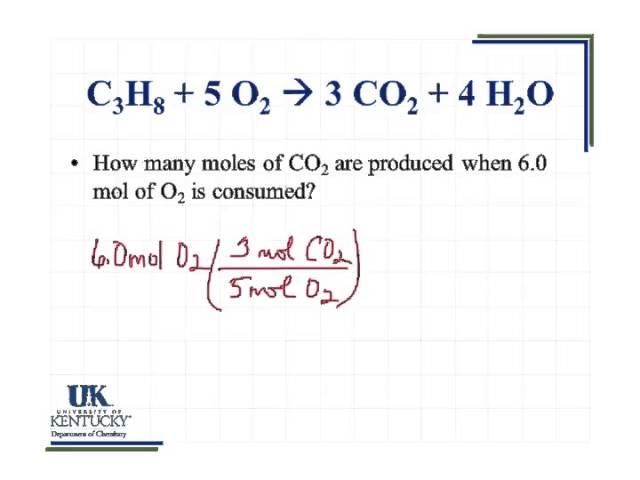 Source: youtube.com
Source: youtube.com
1 mole is equal to 1 moles CO2 or 440095 grams. How many molecules of CO2 are in 1 mole of CO2. How many molecules are in 2 moles of CO2. 1 mole is equal to 1 moles Co2 or 1178664 grams. A mole of CO2 molecules we usually just say a mole of CO2 has one mole of carbon atoms and two moles of oxygen atoms.
 Source: slideplayer.com
Source: slideplayer.com
Use this page to learn how to convert between grams Carbon Dioxide and mole. A mole of CO2 molecules we usually just say a mole of CO2 has one mole of carbon atoms and two moles of oxygen atoms. Number of moles mass molar mass 1g 44 gmol-1 002 moles. Chemistry questions and answers. 1 mole is equal to 1 moles CO2 or 440095 grams.
 Source: numerade.com
Source: numerade.com
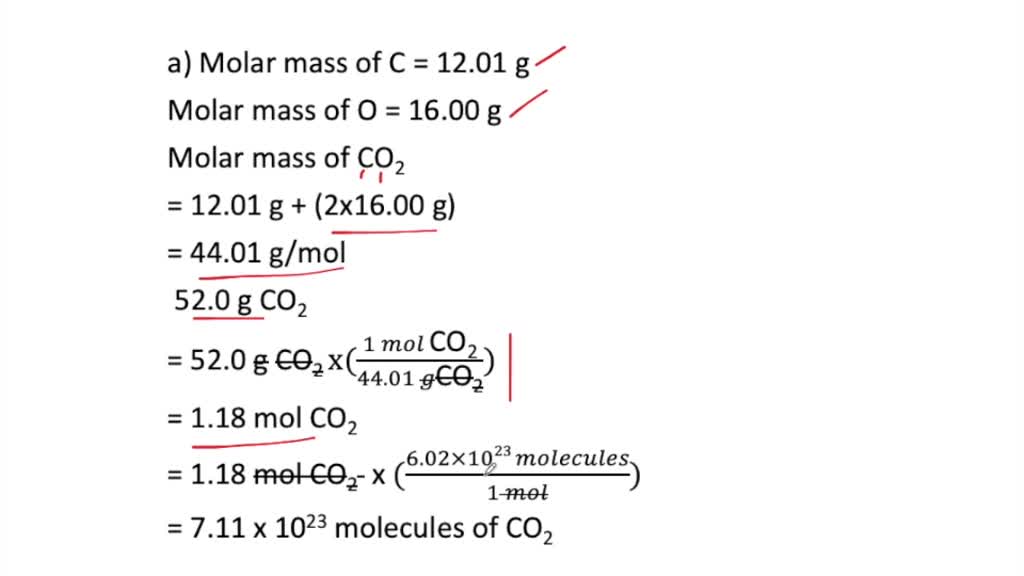
How many molecules are in a 440 g sample of carbon dioxide CO2. 1 6022102360221023 molecules of CO2 are present. 120107159994 2 4401 gmol. Chemistry questions and answers. Where N A Avocado number 6022 1023 mol1.
 Source: youtube.com
Source: youtube.com
1 mole of carbon atoms per mole of carbon dioxide 602 x 1023 carbon atoms. 1 mole carbon dioxide contains 602 x 1023 molecules. How large is a carbon dioxide molecule. There are 6022 X 1023 molecules of CO2 in a mole enough to make the grams in the mole equal the mass. Mr of carbon dioxide is 2161244 Now times by Abogadros constant.

1 grams Carbon Dioxide is equal to 0022722366761722 mole. The mass of one mole of CO2 is 4401 grams. The SI base unit for amount of substance is the mole. Carbon has a molar mass of 120107 gmol and oxygen has a molar mass of 159994 gmol. How many molecules are in 2 moles of CO2.
 Source: pinterest.com
Source: pinterest.com
The number of molecules present in every gas is equal since the molecular mass of carbon dioxide is equal to 44 grams and 44 grams of carbon dioxide consist of these number of molecules. Therefore 22 g of CO2 will contain 3011 x 1023 molecules. Is the mass of one molecule of CO2 while 4401 g is the mass of one mole of CO2 or 6022 x 1023 molecules of CO2 or 3 x 602 x 1023 total number of atoms. This compound is also known as Carbon Dioxide. How many atoms of oxygen are there in one mole of carbon dioxide.
 Source: youtube.com
Source: youtube.com
The number of molecules present in every gas is equal since the molecular mass of carbon dioxide is equal to 44 grams and 44 grams of carbon dioxide consist of these number of molecules. How many molecules of CO2 are in 1 mole of CO2. Molecular weight of CO2 or mol This compound is also known as Carbon Dioxide. And we take the product of the quotient. Of oxygen atoms in 88 g of CO2 contains is 2260221023 atoms.

How many molecules are in a 440 g sample of carbon dioxide CO2. Lets consider the carbon dioxide molecule. Multiplying these values to reflect the proportions in a single molecule of carbon dioxide gives us. So to find the numb of O atoms you do the following. 1 mole of carbon dioxide consist of 6023 x1023 molecules.
 Source: youtube.com
Source: youtube.com
The number of molecules present in every gas is equal since the molecular mass of carbon dioxide is equal to 44 grams and 44 grams of carbon dioxide consist of these number of molecules. 0112 moles CO2 x 6022x10 23 moleculemole x 2 O atomsmolecule number of O atoms. Lets consider the carbon dioxide molecule. How many molecules of CO2 are in 1 mole of CO2. Lets consider the carbon dioxide molecule.

How many molecules are in 2 moles of CO2. Molecular weight of Co2 or grams. 3 21895 x 1023 656879 x 1023 atoms in 16 grams of carbon dioxide. Just so how many molecules are there in 1 mole of co2. Note that rounding errors may occur so always check the results.
This site is an open community for users to do submittion their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site beneficial, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title how many molecules are in one mole of carbon dioxide co2 by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






