Your How many molecules are in 1 mole of oxygen images are available. How many molecules are in 1 mole of oxygen are a topic that is being searched for and liked by netizens now. You can Download the How many molecules are in 1 mole of oxygen files here. Download all free photos.
If you’re searching for how many molecules are in 1 mole of oxygen pictures information linked to the how many molecules are in 1 mole of oxygen interest, you have come to the right blog. Our website frequently provides you with hints for downloading the highest quality video and picture content, please kindly surf and find more informative video content and images that fit your interests.
How Many Molecules Are In 1 Mole Of Oxygen. The number of molecules in 16 gm of oxygen are 05 1632 moles. One atom of sulfur has a mass of 3207 amu. O molecules contains 6022 x 1023 molecules. 1 mole is equal to 1 moles CO2 or 440095 grams.
 Learning Science Chemistry Molar Mass From pinterest.com
Learning Science Chemistry Molar Mass From pinterest.com
One atom of sulfur has a mass of 3207 amu. O molecules contains 6022 x 1023 molecules. The mass of oxygen equal to one mole of oxygen is 15998 grams and the mass of one mole of hydrogen is 1008 g. 1 000 000 000 000. The number of any item in a mole is equal to Avogadros numberor 60221415 1023 items. One mole of oxygen gas which has the formula O2 has a mass of 32 g and contains 602 X 1023 molecules of oxygen but 1204 X 1023 2 X 602 X 1023 atoms because each molecule of oxygen contains two oxygen atoms.
Therefore 1 mole of oxygen gas is actually 1 mole of O2 molecules.
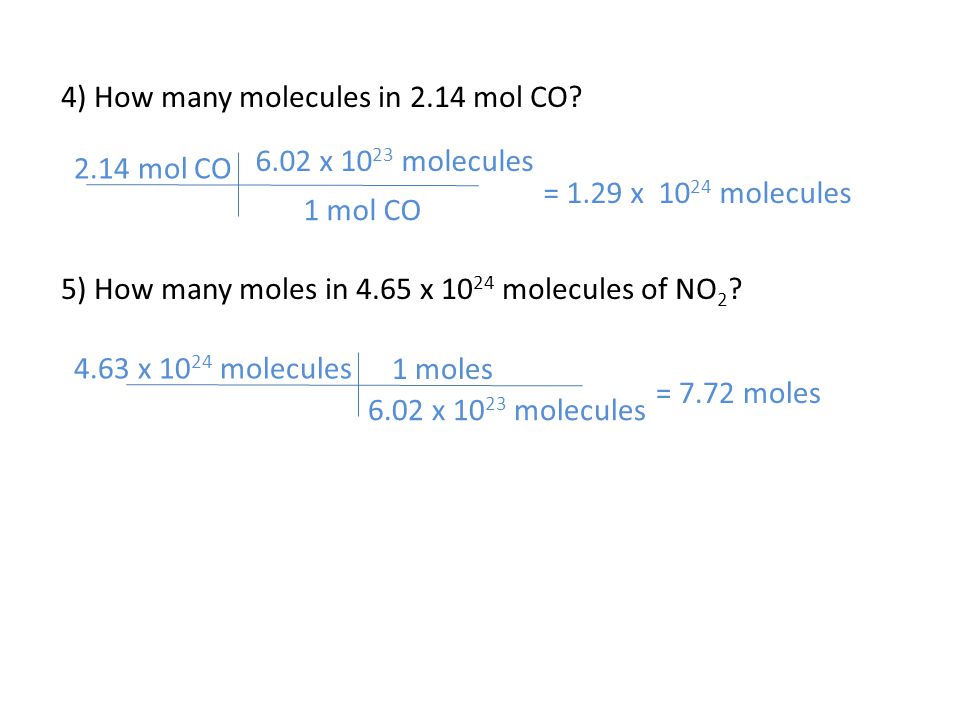
One mole of oxygen gas which has the formula O2 has a mass of 32 g and contains 602 X 1023 molecules of oxygen but 1204 X 1023 2 X 602 X 1023 atoms because each molecule of oxygen contains two oxygen atoms. One mole of oxygen gas which has the formula O2 has a mass of 32 g and contains 602 X 1023 molecules of oxygen but 1204 X 1023 2 X 602 X 1023 atoms because each molecule of oxygen contains two oxygen atoms. One mole of oxygen gas which has the formula O2 has a mass of 32 g and contains 602 X 1023 molecules of oxygen but 1204 X 1023 2 X 602 X 1023 atoms because each molecule of oxygen contains two oxygen atoms. In other words 1 mole of oxygen would contain molecules. I mole contains the Avogadro number 6023 x 1023 molecules O2 So 5 moles would contain 6023x1023 x 5 molecules of O2. Therefore there are 6022 x 1023 atoms of carbon and 12044 x 1023 atoms of oxygen in that 1 mole of CO2.
 Source: pinterest.com
Source: pinterest.com
So in the case of O2 it would be60221415 1023 O2 molecules. There are 6022 1023 molecules in 1 mole of any compound irrespective of elements or the mass of compound. 1 mole is equal to 1 moles Oxygen or 159994 grams. Molecules of oxygen in a mole. I mole contains the Avogadro number 6023 x 1023 molecules O2 So 5 moles would contain 6023x1023 x 5 molecules of O2.
 Source: youtube.com
Source: youtube.com
The molecular formula for Oxygen is O. O molecules contains 6022 x 1023 molecules. Given the number of moles of oxygen gas 01 mol Number of oxygen molecules number of moles N A 01 602 10 23 602 10 22 molecules Based on its formula O 2 each oxygen molecule contains two oxygen atoms. Oxygen weighs 0001429 gram per cubic centimeter or 1429 kilogram per cubic meter ie. Each molecule of O2 is made up of 2 oxygen atoms.
 Source: pinterest.com
Source: pinterest.com
The SI base unit for amount of substance is the mole. The mass of oxygen equal to one mole of oxygen is 15998 grams and the mass of one mole of hydrogen is 1008 g. The SI base unit for amount of substance is the mole. That means that there are two moles of oxygen atoms in a mole of O2 or 12046E24 of them. The number of any item in a mole is equal to Avogadros numberor 60221415 1023 items.
 Source: pinterest.com
Source: pinterest.com
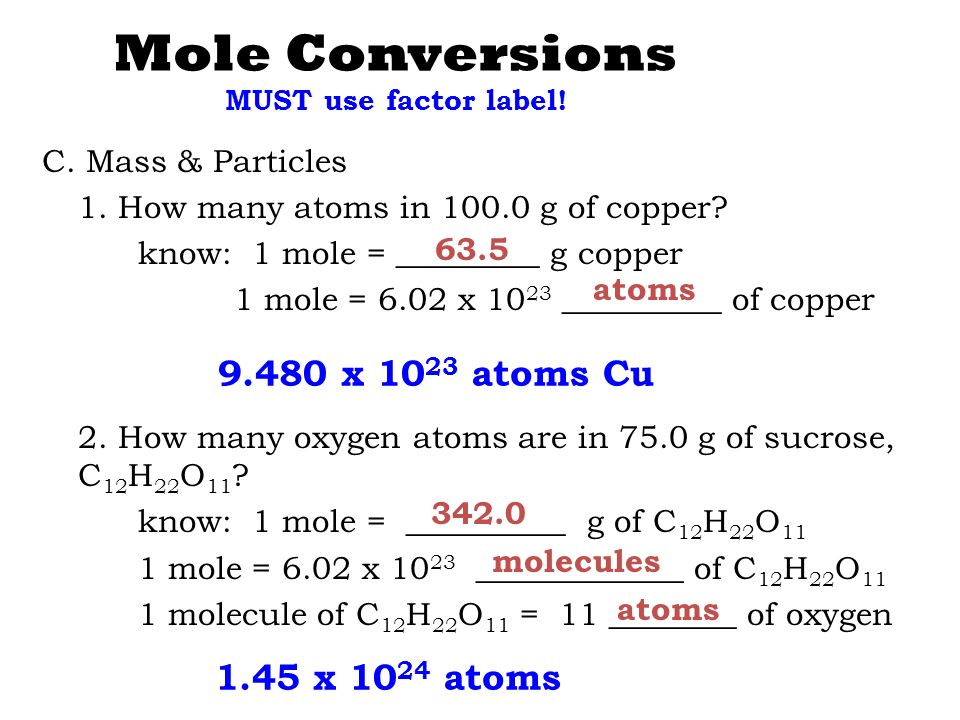
Moles of a Substance and the Molecular Weight. Here we are given with 16 g of oxygen. Avogadros number shows us that there are 6022 x 1023 molecules of CO2 in 1 mole of the gas. So in the case of O2 it would be60221415 1023 O2 molecules. One mole of oxygen gas which has the formula O2 has a mass of 32 g and contains 602 X 1023 molecules of oxygen but 1204 X 1023 2 X 602 X 1023 atoms because each molecule of oxygen contains two oxygen atoms.
 Source: slideplayer.com
Source: slideplayer.com
Oxygen weighs 0001429 gram per cubic centimeter or 1429 kilogram per cubic meter ie. One mole of S atoms has a mass of 3207 g. What is the mole of oxygen. The number of molecules in 16 gm of oxygen are 05 1632 moles. O molecules contains 6022 x 1023 molecules.
 Source: youtube.com
Source: youtube.com
The number of any item in a mole is equal to Avogadros numberor 60221415 1023 items. Molecular weight of Oxygen or grams. So in the case of O2 it would be60221415 1023 O2 molecules. Thus the number of molecules in 16 gm or 05 mole of oxygen gas are 05 x. One atom of sulfur has a mass of 3207 amu.
 Source: slideplayer.com
Source: slideplayer.com
How many atoms of C are in 1 mole of co2. One mole of oxygen gas which has the formula O2 has a mass of 32 g and contains 602 X 1023 molecules of oxygen but 1204 X 1023 2 X 602 X 1023 atoms because each molecule of oxygen contains two oxygen atoms. Density of oxygen is equal to 1429 kgm³. That means that there are two moles of oxygen atoms in a mole of O2 or 12046E24 of them. So 1 mole of O2 molecules is made up of 2 moles of oxygen atoms.
 Source: pinterest.com
Source: pinterest.com
The SI base unit for amount of substance is the mole. Moles of a Substance and the Molecular Weight. So 1 mole of O2 molecules is made up of 2 moles of oxygen atoms. 1 mole contains 6022 x 1023 entities Avogadros number Concept 1. How many atoms of C are in 1 mole of co2.
 Source: slideplayer.com
Source: slideplayer.com
What is the mole of oxygen. If we total up the gram amounts of each element in the water molecule 15998gmol 21008gmol we get the molar mass of water 18014gmol. The number of molecules in 16 gm of oxygen are 05 1632 moles. 1 000 000 000 000. So in the case of O2 it would be60221415 1023 O2 molecules.
 Source: slideplayer.com
Source: slideplayer.com
Therefore there are 6022 x 1023 atoms of carbon and 12044 x 1023 atoms of oxygen in that 1 mole of CO2. At 0C 32F or 27315K at standard atmospheric pressure. How many moles of oxygen are in 1 mole. Thus the number of molecules in 16 gm or 05 mole of oxygen gas are 05 x. 1 000 000 000 000.
 Source: slideplayer.com
Source: slideplayer.com
The number of molecules in 16 gm of oxygen are 05 1632 moles. 1 mole is equal to 1 moles CO2 or 440095 grams. Avogadros number shows us that there are 6022 x 1023 molecules of CO2 in 1 mole of the gas. 1 000 000 000 000. One mole of oxygen gas which has the formula O2 has a mass of 32 g and contains 602 X 1023 molecules of oxygen but 1204 X 1023 2 X 602 X 1023 atoms because each molecule of oxygen contains two oxygen atoms.
 Source: pinterest.com
Source: pinterest.com
So in the case of O2 it would be60221415 1023 O2 molecules. One atom of sulfur has a mass of 3207 amu. One mole of S atoms has a mass of 3207 g. A mole of O2 molecules has 6023E23 molecules and each molecule is made up is two atoms of oxygen. The number of molecules in 16 gm of oxygen are 05 1632 moles.
 Source: pinterest.com
Source: pinterest.com
O molecules contains 6022 x 1023 molecules. Avogadros number shows us that there are 6022 x 1023 molecules of CO2 in 1 mole of the gas. 1 mole is equal to 1 moles Oxygen or 159994 grams. What is the mole of oxygen. Thus in 3 moles of O2 there are 3 6022 1023 molecules of O2 or 180661023 molecules of O2.
 Source: ar.pinterest.com
Source: ar.pinterest.com
Oxygen weighs 0001429 gram per cubic centimeter or 1429 kilogram per cubic meter ie. One mole of oxygen gas which has the formula O2 has a mass of 32 g and contains 602 X 1023 molecules of oxygen but 1204 X 1023 2 X 602 X 1023 atoms because each molecule of oxygen contains two oxygen atoms. Molecular weight of Oxygen or grams. One mole of oxygen gas which has the formula O2 has a mass of 32 g and contains 602 X 1023 molecules of oxygen but 1204 X 1023 2 X 602 X 1023 atoms because each molecule of oxygen contains two oxygen atoms. So in the case of O2 it would be60221415 1023 O2 molecules.
 Source: slidetodoc.com
Source: slidetodoc.com
180661023 molecules of O2. So 1 mole of O2 molecules is made up of 2 moles of oxygen atoms. Moles of a Substance and the Molecular Weight. One mole of Water is composed of 1 mole of Oxygen and two moles of Hydrogen. One mole of oxygen gas which has the formula O2 has a mass of 32 g and contains 602 X 1023 molecules of oxygen but 1204 X 1023 2 X 602 X 1023 atoms because each molecule of oxygen contains two oxygen atoms.
This site is an open community for users to do submittion their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site beneficial, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title how many molecules are in 1 mole of oxygen by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






