Your How many molecules are in 1 mole of h2s images are available. How many molecules are in 1 mole of h2s are a topic that is being searched for and liked by netizens today. You can Download the How many molecules are in 1 mole of h2s files here. Download all free images.
If you’re searching for how many molecules are in 1 mole of h2s images information linked to the how many molecules are in 1 mole of h2s keyword, you have come to the right blog. Our site always gives you hints for seeking the maximum quality video and picture content, please kindly search and locate more enlightening video content and images that match your interests.
How Many Molecules Are In 1 Mole Of H2s. However as Table PageIndex1 shows ceO2 is twice as soluble in water as ceN2. When H2S forms each H atom shares their 1e with S and in doing so has achieved a stable duet. Succinic acid an intermediate in the metabolism of food molecules has molecular mass 1181 amu. Nitrogen and oxygen are the two most prominent gases in the Earths atmosphere and they share many similar physical properties.
 In The Given Reaction H2s G So2 G S S H2o One Equivalent Of H2s G Will Reduce From toppr.com
In The Given Reaction H2s G So2 G S S H2o One Equivalent Of H2s G Will Reduce From toppr.com
When 1926 g of succinic acid was dissolved in water and titrated 6520 mL of. Each H atom in a compound can contribute 1 valence e a S atom contributes 6 valence e. The conversion from one unit to another is based on the Law of Avogadros according to which equal volumes of gases at the same temperature and pressure contain the same number of molecules. The density of butane is 06011 gmL and the molar mass is 5812 gmol. Nitrogen and oxygen are the two most prominent gases in the Earths atmosphere and they share many similar physical properties. According to the first corollary from Avogadros law one mole of any gas under the same - isobaric and isothermal - conditions occupies the same volume.
A 259 1023 molecules butane B 146 1027 molecules butane C 687 1023 molecules butane D 156 1023 molecules butane E 714 1025 molecules butane.
Each H atom in a compound can contribute 1 valence e a S atom contributes 6 valence e. However as Table PageIndex1 shows ceO2 is twice as soluble in water as ceN2. The S atom started with 6 valence electrons and upon. A 259 1023 molecules butane B 146 1027 molecules butane C 687 1023 molecules butane D 156 1023 molecules butane E 714 1025 molecules butane. When H2S forms each H atom shares their 1e with S and in doing so has achieved a stable duet. According to the first corollary from Avogadros law one mole of any gas under the same - isobaric and isothermal - conditions occupies the same volume.
 Source: slideplayer.com
Source: slideplayer.com
Many factors contribute to solubility including the nature of the intermolecular forces at play. Nitrogen and oxygen are the two most prominent gases in the Earths atmosphere and they share many similar physical properties. A 259 1023 molecules butane B 146 1027 molecules butane C 687 1023 molecules butane D 156 1023 molecules butane E 714 1025 molecules butane. Many factors contribute to solubility including the nature of the intermolecular forces at play. The S atom started with 6 valence electrons and upon.
 Source: brainly.in
Source: brainly.in
However as Table PageIndex1 shows ceO2 is twice as soluble in water as ceN2. The S atom started with 6 valence electrons and upon. Each H atom in a compound can contribute 1 valence e a S atom contributes 6 valence e. The conversion from one unit to another is based on the Law of Avogadros according to which equal volumes of gases at the same temperature and pressure contain the same number of molecules. When H2S forms each H atom shares their 1e with S and in doing so has achieved a stable duet.
 Source: brainly.in
Source: brainly.in
However as Table PageIndex1 shows ceO2 is twice as soluble in water as ceN2. However as Table PageIndex1 shows ceO2 is twice as soluble in water as ceN2. Nitrogen and oxygen are the two most prominent gases in the Earths atmosphere and they share many similar physical properties. The conversion from one unit to another is based on the Law of Avogadros according to which equal volumes of gases at the same temperature and pressure contain the same number of molecules. According to the first corollary from Avogadros law one mole of any gas under the same - isobaric and isothermal - conditions occupies the same volume.
 Source: toppr.com
Source: toppr.com
Many factors contribute to solubility including the nature of the intermolecular forces at play. Each H atom in a compound can contribute 1 valence e a S atom contributes 6 valence e. However as Table PageIndex1 shows ceO2 is twice as soluble in water as ceN2. When H2S forms each H atom shares their 1e with S and in doing so has achieved a stable duet. The density of butane is 06011 gmL and the molar mass is 5812 gmol.
 Source: jbc.org
Source: jbc.org
A 259 1023 molecules butane B 146 1027 molecules butane C 687 1023 molecules butane D 156 1023 molecules butane E 714 1025 molecules butane. The density of butane is 06011 gmL and the molar mass is 5812 gmol. A 259 1023 molecules butane B 146 1027 molecules butane C 687 1023 molecules butane D 156 1023 molecules butane E 714 1025 molecules butane. The S atom started with 6 valence electrons and upon. Succinic acid an intermediate in the metabolism of food molecules has molecular mass 1181 amu.
 Source: meritnation.com
Source: meritnation.com
The conversion from one unit to another is based on the Law of Avogadros according to which equal volumes of gases at the same temperature and pressure contain the same number of molecules. Many factors contribute to solubility including the nature of the intermolecular forces at play. The density of butane is 06011 gmL and the molar mass is 5812 gmol. When H2S forms each H atom shares their 1e with S and in doing so has achieved a stable duet. Nitrogen and oxygen are the two most prominent gases in the Earths atmosphere and they share many similar physical properties.
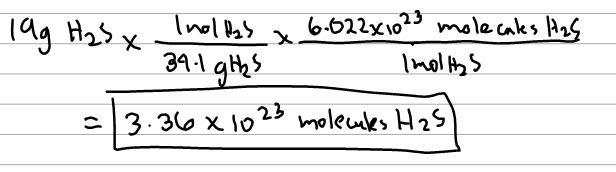 Source: clutchprep.com
Source: clutchprep.com
Nitrogen and oxygen are the two most prominent gases in the Earths atmosphere and they share many similar physical properties. However as Table PageIndex1 shows ceO2 is twice as soluble in water as ceN2. The S atom started with 6 valence electrons and upon. Nitrogen and oxygen are the two most prominent gases in the Earths atmosphere and they share many similar physical properties. When H2S forms each H atom shares their 1e with S and in doing so has achieved a stable duet.

How many molecules are contained in 250 mL of butane. Many factors contribute to solubility including the nature of the intermolecular forces at play. When H2S forms each H atom shares their 1e with S and in doing so has achieved a stable duet. The S atom started with 6 valence electrons and upon. According to the first corollary from Avogadros law one mole of any gas under the same - isobaric and isothermal - conditions occupies the same volume.
 Source: researchgate.net
Source: researchgate.net
A 259 1023 molecules butane B 146 1027 molecules butane C 687 1023 molecules butane D 156 1023 molecules butane E 714 1025 molecules butane. According to the first corollary from Avogadros law one mole of any gas under the same - isobaric and isothermal - conditions occupies the same volume. How many molecules are contained in 250 mL of butane. The density of butane is 06011 gmL and the molar mass is 5812 gmol. A 259 1023 molecules butane B 146 1027 molecules butane C 687 1023 molecules butane D 156 1023 molecules butane E 714 1025 molecules butane.
 Source: toppr.com
Source: toppr.com
The density of butane is 06011 gmL and the molar mass is 5812 gmol. When H2S forms each H atom shares their 1e with S and in doing so has achieved a stable duet. The S atom started with 6 valence electrons and upon. Each H atom in a compound can contribute 1 valence e a S atom contributes 6 valence e. The density of butane is 06011 gmL and the molar mass is 5812 gmol.
 Source: researchgate.net
Source: researchgate.net
However as Table PageIndex1 shows ceO2 is twice as soluble in water as ceN2. When 1926 g of succinic acid was dissolved in water and titrated 6520 mL of. When H2S forms each H atom shares their 1e with S and in doing so has achieved a stable duet. The conversion from one unit to another is based on the Law of Avogadros according to which equal volumes of gases at the same temperature and pressure contain the same number of molecules. The density of butane is 06011 gmL and the molar mass is 5812 gmol.
 Source: pinterest.com
Source: pinterest.com
Many factors contribute to solubility including the nature of the intermolecular forces at play. The density of butane is 06011 gmL and the molar mass is 5812 gmol. When 1926 g of succinic acid was dissolved in water and titrated 6520 mL of. Succinic acid an intermediate in the metabolism of food molecules has molecular mass 1181 amu. However as Table PageIndex1 shows ceO2 is twice as soluble in water as ceN2.
 Source: researchgate.net
Source: researchgate.net
Nitrogen and oxygen are the two most prominent gases in the Earths atmosphere and they share many similar physical properties. A 259 1023 molecules butane B 146 1027 molecules butane C 687 1023 molecules butane D 156 1023 molecules butane E 714 1025 molecules butane. How many molecules are contained in 250 mL of butane. Each H atom in a compound can contribute 1 valence e a S atom contributes 6 valence e. Many factors contribute to solubility including the nature of the intermolecular forces at play.
 Source: researchgate.net
Source: researchgate.net
Succinic acid an intermediate in the metabolism of food molecules has molecular mass 1181 amu. Nitrogen and oxygen are the two most prominent gases in the Earths atmosphere and they share many similar physical properties. Many factors contribute to solubility including the nature of the intermolecular forces at play. How many molecules are contained in 250 mL of butane. The conversion from one unit to another is based on the Law of Avogadros according to which equal volumes of gases at the same temperature and pressure contain the same number of molecules.
 Source: pinterest.com
Source: pinterest.com
When H2S forms each H atom shares their 1e with S and in doing so has achieved a stable duet. Nitrogen and oxygen are the two most prominent gases in the Earths atmosphere and they share many similar physical properties. According to the first corollary from Avogadros law one mole of any gas under the same - isobaric and isothermal - conditions occupies the same volume. Many factors contribute to solubility including the nature of the intermolecular forces at play. The density of butane is 06011 gmL and the molar mass is 5812 gmol.
This site is an open community for users to do submittion their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site helpful, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title how many molecules are in 1 mole of h2s by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






