Your How many molecules are in 1 mole of h2o2 images are available. How many molecules are in 1 mole of h2o2 are a topic that is being searched for and liked by netizens today. You can Find and Download the How many molecules are in 1 mole of h2o2 files here. Download all royalty-free images.
If you’re looking for how many molecules are in 1 mole of h2o2 pictures information linked to the how many molecules are in 1 mole of h2o2 interest, you have come to the ideal blog. Our website always provides you with suggestions for viewing the highest quality video and picture content, please kindly search and find more enlightening video content and images that fit your interests.
How Many Molecules Are In 1 Mole Of H2o2. 68gH2O2 36 g H2O 32g O2 1 mole of any covalent compound 602 x10 23 molecules. Im sure that you remember Avogadros Number by Amadeo Carlo Avogadro 1776 - 1856 which states that 1 mole of a substance will contain 6022 x 1023 molecules. So hydrogen peroxide has a molecular weight of 340146 grams per mole. According to the formula there are 2 moles of hydrogen and 2 moles of oxygen in each hydrogen peroxide molecule.
 Mahjong Chem Free Game To Practice Chemistry Knowledge Chemistry Chemistry Lessons Classroom Technology From sk.pinterest.com
Mahjong Chem Free Game To Practice Chemistry Knowledge Chemistry Chemistry Lessons Classroom Technology From sk.pinterest.com
So the number of moles of H atom in it. The meaning and usefulness of the mole. The SI base unit for amount of substance is the mole. 2moles H2O2 2 moles H2O 1 mole O2 Mass -mass relationship. One mole of H 2 O molecules contains 6022 x 10 23 molecules. Its atomic number is 8.
The molar mass of H2O2 is 3401 gmol.
Assuming a density of 10 gmL calculate the molarity and mole fraction of 30 h2o2. Hydrogen H Oxygen O Molecular weight. There are 602E23 molecules in a mol of moleculesso you can convert. Assuming a density of 10 gmL calculate the molarity and mole fraction of 30 h2o2. The molecular formula for Hydrogen Peroxide is H2O2. Oxygens atomic mass is 15999 amu.
 Source: doubtnut.com
Source: doubtnut.com
68gH2O2 36 g H2O 32g O2 1 mole of any covalent compound 602 x10 23 molecules. 11 atoms1 molecule C3H8 3. You can view more similar questions or ask a new question. Its atomic number is 8. We assume you are converting between grams H2O2 and mole.

Molecular weight of H2O2 or mol. What number of molecules of H2O2 are in 0350 L of hydrogen peroxide. This compound is also known as Hydrogen Peroxide. Mar 13 2017. So 1 mole of CH 4 will have 4 mol atom of Hydrogen.
 Source: pinterest.com
Source: pinterest.com
There are 602E23 molecules in a mol of moleculesso you can convert. And each molecule contains 4. The molar mass of H2O2 is 3401 gmol. Molecular weight of Hydrogen Peroxide or grams. 1 grams H2O2 is equal to 0029399071224542 mole.

The answer is 3401468. Mar 13 2017. The definition of Avogadros number of 6022 1023mole is the number of atoms or molecules per one gram atomic weight. 2moles H2O2 2 moles H2O 1 mole O2 Mass -mass relationship. The SI base unit for amount of substance is the mole.
 Source: youtube.com
Source: youtube.com
Mass 350 mL x 145 gmL 5075 g mols H2O2 in 5075 g 50753401 149 mols. The molecular formula for Hydrogen Peroxide is H2O2. 212 mol C3H8 X 6022 X 1023 1 mol C3H8 X 11 atoms1 molecule C3H8 Answer 140 X 1025 atoms. How many atoms of hydrogen are in 3 moles of hydrogen peroxide. What number of molecules of H2O2 are in 0350 L of hydrogen peroxide.
 Source: pinterest.com
Source: pinterest.com
But each molecule of water contains 2 H and 1 O atom 3 atoms so there are approximately 18 x. The molecular mass of H2O2 is 210 2160 340Amount ofH2O2 6802340 0200molSo there are 0200 moles of H2O2 moles. This compound is also known as Hydrogen Peroxide. How many molecules of hydrogen gas H2 are in an identical container at the same temperature and pressure. For one gram atomic weight of hydrogen with atomic weight of one gram one mole of hydrogen contains 6022 1023 hydrogen atoms.
 Source: toppr.com
Source: toppr.com
Boiling Point BP Hydrogen peroxide changes its state from liquid to gas at 1502C 30236F or 42335K Also known as. How many atoms of hydrogen are in 3 moles of hydrogen peroxide. Hydrogen H Oxygen O Molecular weight. How many atoms are in 212 mol of propane C3H8 1. You can view more details on each measurement unit.
 Source: sk.pinterest.com
Source: sk.pinterest.com
According to the formula there are 2 moles of hydrogen and 2 moles of oxygen in each hydrogen peroxide molecule. Now you want to know how many molecules are present in 3 moles of hydrogen sulfide H2S. 1 mole contains 6022 x 10 23 entities Avogadros number Concept 1. Its atomic number is 8. According to the formula there are 2 moles of hydrogen and 2 moles of oxygen in each hydrogen peroxide molecule.
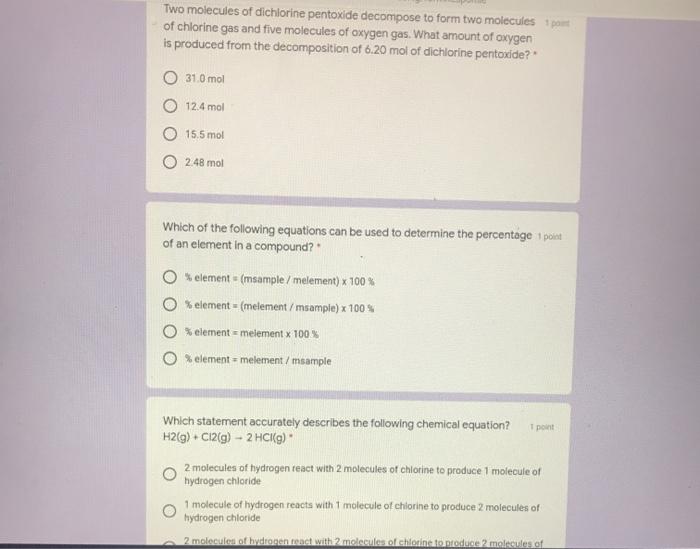
The SI base unit for amount of substance is the mole. Molecular weight of H2O2 or grams This compound is often known as Hydrogen Peroxide. The SI base unit for amount of substance is the mole. BaBr2 Ca - CaBr2 Ba OE 2LIBr Ba - BaBrą 2L1. 1 mole contains 6022 x 10 23 entities Avogadros number Concept 1.
 Source: researchgate.net
Source: researchgate.net
How many moles of NH3 can be produced from 550 moles of nitrogen in the following reaction. The density of hydrogen peroxide H2O2 is 145 gmL. What number of molecules of H2O2 are in 0350 L of hydrogen peroxide. An Introduction to Physical Science 14th Edition Edit edition Solutions for Chapter 13 Problem 11MC. It is found that Kt makes up 950 of the entire mass of the sample.

Its atomic number is 8. One mole of hydrogen peroxide contains 602 x 1023 molecules of hydrogen peroxide. 1 grams H2O2 is equal to 0029399071224542 mole. Assuming a density of 10 gmL calculate the molarity and mole fraction of 30 h2o2. One mole of water contains 602 x 1023 MOLECULES of water.
 Source: slideplayer.com
Source: slideplayer.com
Mass 350 mL x 145 gmL 5075 g mols H2O2 in 5075 g 50753401 149 mols. 2moles H2O2 2 moles H2O 1 mole O2 Mass -mass relationship. 68gH2O2 36 g H2O 32g O2 1 mole of any covalent compound 602 x10 23 molecules. To get the exact number multiply this by the Avogadrosconstant. This compound is also known as Hydrogen Peroxide.

2moles H2O2 2 moles H2O 1 mole O2 Mass -mass relationship. The meaning and usefulness of the mole. 212 mol C3H8 X 6022 X 1023 1 mol C3H8 X 11 atoms1 molecule C3H8 Answer 140 X 1025 atoms. The molecular mass of H2O2 is 210 2160 340Amount ofH2O2 6802340 0200molSo there are 0200 moles of H2O2 moles. Its atomic number is 8.

The molar mass of H2O2 is 3401 gmol. The molecular mass of H2O2 is 210 2160 340Amount ofH2O2 6802340 0200molSo there are 0200 moles of H2O2 moles. 11 atoms1 molecule C3H8 3. Oxygens atomic mass is 15999 amu. Mass 350 mL x 145 gmL 5075 g mols H2O2 in 5075 g 50753401 149 mols.
 Source: toppr.com
Source: toppr.com
The SI base unit for amount of substance is the mole. How many moles of hydrogen peroxide H2O2 molecules are there in 900x109 9Billion H2O2 molecules. As hydrogen gas is diatomic 3 moles of hydrogen gas contains 3 x 6022 x 1023 H2 molecules each containing 2 hydrogen atoms. So the number of moles of H atom in it. And each molecule contains 4.
This site is an open community for users to do sharing their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site adventageous, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title how many molecules are in 1 mole of h2o2 by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






