Your How many molecules are in 1 mole of calcium carbonate images are ready in this website. How many molecules are in 1 mole of calcium carbonate are a topic that is being searched for and liked by netizens today. You can Get the How many molecules are in 1 mole of calcium carbonate files here. Download all royalty-free photos.
If you’re searching for how many molecules are in 1 mole of calcium carbonate pictures information related to the how many molecules are in 1 mole of calcium carbonate keyword, you have come to the right site. Our site frequently provides you with hints for seeking the highest quality video and picture content, please kindly search and find more enlightening video articles and graphics that match your interests.
How Many Molecules Are In 1 Mole Of Calcium Carbonate. We assume you are converting between grams Calcium Carbonate and mole. So there will be 01 moles of calcium carbonate in this sample. Of moleculesAvogadro constant 6022 1023 6022 1023 1. It has a molar mass of around 100 gmol.
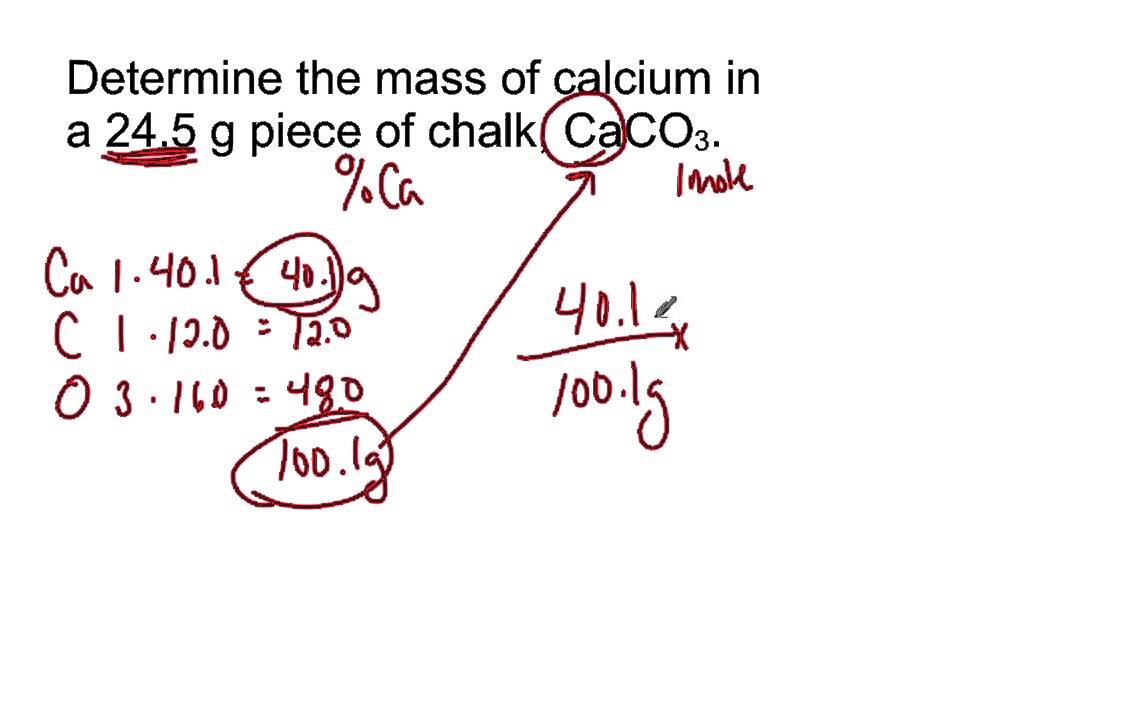 Mass Of Ca In 24 5 G Sample Of Calcium Carbonate Youtube From youtube.com
Mass Of Ca In 24 5 G Sample Of Calcium Carbonate Youtube From youtube.com
How many moles are in CaCO3. 1gram of CaCO31100 mole. The molecular formula for Calcium Carbonate is CaCO3. 10gram of CaCO311001001 mole. How many molecules of calcium carbonate are present in its 1 mole. The chemical formula for CaCO₃ 100086 g.
100 g of Calcium Carbonate 100000 mg of Calcium Carbonate has 3 moles of Oxygen atoms 3 x 6022 x 1023 Oxygen atoms.
And thus we say that there are 450molxxN_A formula units of. Of moleculesAvogadro constant 6022 1023 6022 1023 1. One mole of calcium carbonate CaCO3 weighs 100 units. 1 u is equal to 112 the mass of one atom of carbon-12 Molar mass molar weight is the mass of one mole of a substance and is expressed in gmol. Therefore if the question is how many moles of oxygen atoms are there in one mole of calcium carbonate the answer would be three. How many moles are 5 gram of calcium.
 Source: brainly.in
Source: brainly.in
Convert grams CaCO3 to moles or moles CaCO3 to grams. So there will be 01 moles of calcium carbonate in this sample. 1 mole is equal to 1 moles NH42CO3 or 9608582 grams. 10gram of CaCO311001001 mole. So we need to determine how many molecules of calcium carbonate are present in 1100 mg.
 Source: youtube.com
Source: youtube.com
However oxygen as it is commonly known is typically found as the molecule O₂ so if the oxygen atoms were removed from the CaCO₃ there would only be one and a half moles of oxygen the gas. Of moles of CaCO3 No. 10g 100gmol 01 mol. The SI base unit for amount of substance is the mole. Each mole of H 2O molecules contains 2 moles of H and 1 mole of O One mole of O atoms corresponds to 159994 g.

1 x Ca40 2 x N14 6 x O16 164grams per mole 164gmol. One mole of calcium carbonate CaCO3 weighs 100 units. Solution Molar mass Molecular mass in gram of CaCO3 4012316 100 g No. Calcium Nitrate is Ca NO32. Ca 40 u C 12 u O 16 u.

Therefore 01 mole of CaCO3 is present in 10grams of the substance. Calculate the percent of composition of vitamin C. The number of molecules in one mole of a substance is given by the Avogadros number 6022 10²³ molecules. The number of calcium atoms in one molecule of CaCO₃ 1 atom of calcium. As one mole of molecules is defined by 60221023 molecules.
 Source: nagwa.com
Source: nagwa.com
The SI base unit for amount of substance is the mole. 100 g of Calcium Carbonate 100000 mg of Calcium Carbonate has 3 moles of Oxygen atoms 3 x 6022 x 1023 Oxygen atoms. Molecules of CaCo3 160231023. It has a molar mass of around 100 gmol. So there will be 01 moles of calcium carbonate in this sample.
 Source: youtube.com
Source: youtube.com
As one mole of molecules is defined by 60221023 molecules. Numerical problems based On Mole Concept Calculate the mass of 6022 1023 molecule of Calcium carbonate CaCO3. You can view more details on each measurement unit. 1gram of CaCO31100 mole. 100 g of Calcium Carbonate 100000 mg of Calcium Carbonate has 3 moles of Oxygen atoms 3 x 6022 x 1023 Oxygen atoms.
 Source: toppr.com
Source: toppr.com
Well calcium carbonate or limestone has a chemical formula of CaCO3. The SI base unit for amount of substance is the mole. 10gram of CaCO311001001 mole. To do so we need to look up the molar mass of calcium carbonate which is listed as 1000869 gmol. Therefore 01 mole of CaCO3 is present in 10grams of the substance.
 Source: slideplayer.com
Source: slideplayer.com
Of moleculesAvogadro constant 6022 1023 6022 1023 1. Ca 40 C 12 O 16 Solve Study Textbooks. 1gram of CaCO31100 mole. Molecular weight of Calcium Carbonate or mol The molecular formula for Calcium Carbonate is CaCO3. How many moles are in CaCO3.

Bezglasnaaz and 283 more users found this answer. Now calcium carbonate is NOT MOLECULAR. 1 mole is equal to 1 moles NH42CO3 or 9608582 grams. Ca 40 C 12 O 16 Solve Study Textbooks. Convert grams CaCO3 to moles or moles CaCO3 to grams.
 Source: youtube.com
Source: youtube.com
Therefore if the question is how many moles of oxygen atoms are there in one mole of calcium carbonate the answer would be three. One mole of calcium carbonate CaCO3 weighs 100 units. For our case we can say that there is 100g of CaCO3 in one mole of the substance. Bezglasnaaz and 283 more users found this answer. How many formula units are present if the substance is calcium carbonate CaCO₃.
 Source: brainly.in
Source: brainly.in
Of moleculesAvogadro constant 6022 1023 6022 1023 1. 275100 yields 0275 moles. The molecular formula for Calcium Carbonate is CaCO3. Calculate the percent of composition of vitamin C. How many grams Calcium Carbonate in 1 mol.
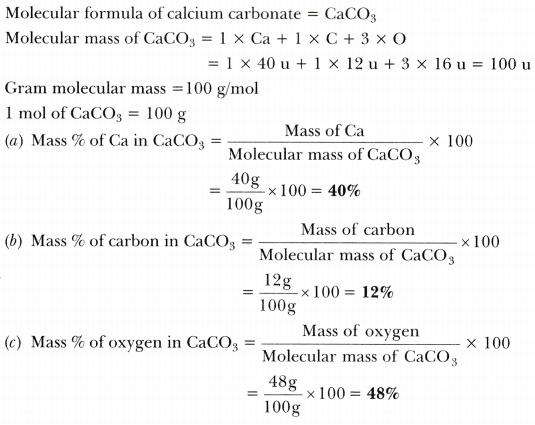 Source: ask.learncbse.in
Source: ask.learncbse.in
Calcium carbonate CaCO 3 1 Ca 40078 1 C 12011 3 O 3 x 159994 Molar mass of CaCO 3 100087 g IronII sulfate FeSO 4 Molar mass of FeSO 4 151909 g Chapter 3. 1 x Ca40 2 x N14 6 x O16 164grams per mole 164gmol. We can also use the abbreviation N_A6022xx1023mol-1. So we need to determine how many molecules of calcium carbonate are present in 1100 mg. 1 mole is equal to 1 moles Calcium Carbonate or 1000869 grams.
 Source: toppr.com
Source: toppr.com
You can view more details on each measurement unit. How many moles are 5 gram of calcium. 1 mole of CaCO3 contains one mole of Calcium one mole of Carbon and 3 moles of Oxygen. Of moleculesAvogadro constant 6022 1023 6022 1023 1. Click hereto get an answer to your question How many moles of calcium carbonate CaCO3 are present in 20 g of the substance.
 Source: slideplayer.com
Source: slideplayer.com
We assume you are converting between grams Calcium Carbonate and mole. The SI base unit for amount of substance is the mole. How many moles are 5 gram of calcium. How many grams Calcium Carbonate in 1 mol. To do so we need to look up the molar mass of calcium carbonate which is listed as 1000869 gmol.
 Source: youtube.com
Source: youtube.com
Molecules of CaCo3 160231023. Grams can be canceled leaving the moles of K. Assume you are given 044 mol of a substance. The number of calcium atoms in one molecule of CaCO₃ 1 atom of calcium. So we need to determine how many molecules of calcium carbonate are present in 1100 mg.
This site is an open community for users to do submittion their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site adventageous, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title how many molecules are in 1 mole of calcium carbonate by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






