Your How many hydrogen atoms in 1 mole of water images are ready in this website. How many hydrogen atoms in 1 mole of water are a topic that is being searched for and liked by netizens now. You can Get the How many hydrogen atoms in 1 mole of water files here. Get all free vectors.
If you’re looking for how many hydrogen atoms in 1 mole of water images information linked to the how many hydrogen atoms in 1 mole of water topic, you have pay a visit to the ideal blog. Our website always provides you with suggestions for seeing the maximum quality video and picture content, please kindly search and find more informative video content and images that match your interests.
How Many Hydrogen Atoms In 1 Mole Of Water. How many atoms of hydrogen are there in 18 g of water. Click to see full answer In respect to this how many hydrogen atoms are there in 300 mole of h2o. 52 1024 Avogadros number. From the periodic table we see the atomic weight of hydrogen is.

In one mole of. 52 1024 Avogadros number. The chemical formula for aluminum hydroxide is AlOH3. So the number of moles of H atom in it. Dual that for 2 moles as well as there are 24088 x 10 23 hydrogen atoms in one mole of water. So 1 mole H2O 120441024 hydrogen atoms.
If we total up the gram amounts of each element in the water molecule 15998gmol 21008gmol we get the molar mass of water 18014gmol.
Mar 13 2017. Answer 1 of 5. How many grams are in 15 moles of water. For each mole of water there are two moles of H and one mole of O. You have 314 moles of water. The mass of oxygen equal to one mole of oxygen is 15998 grams and the mass of one mole of hydrogen is 1008 g.
 Source: slideplayer.com
Source: slideplayer.com
A mole of water molecules contains 2 moles of hydrogen atoms and 1 mole of oxygen atoms. In one mole of. 1 mole of water contains 602310 23 molecules of water or. Youd go about calculating this by using Avogadros number which links the number of atoms or molecules of a substance with the number of moles of that substance you have. One may also ask how many moles of hydrogen are in h2o.
 Source: pinterest.com
Source: pinterest.com
The one mole of any substance is equal to the 6023 times 1023 units of atoms molecules or ions. 120 g of water corresponds to 1812. This indicates that in one mole of aluminum hydroxide there are three moles of hydrogen atoms. 20 g of hydrogen gas is 10 moles of H2 which is 20 moles of H atoms. Click to see full answer In respect to this how many hydrogen atoms are there in 300 mole of h2o.
 Source: pinterest.com
Source: pinterest.com
Because the mole contains so many units theyre most often used in chemistry is a way of measuring really really small things like atoms or molecules. Similarly how many hydrogen atoms are in a mole of water. 100g of water is 556 moles of water which would have 1112 moles of H atoms. Also know how many hydrogen atoms are there in 300 mole of h2o. 52 1024 Avogadros number.
 Source: quora.com
Source: quora.com
In one mole of water there will exist approximately 6021023 water molecules. So there will be a total of 60210232121024 hydrogen atoms. Double that for 2 moles and there are 24088 x 1023 hydrogen atoms in one mole of water. A mole of water molecules contains 2 moles of hydrogen atoms and 1 mole of oxygen atoms. Click to see full answer In respect to this how many hydrogen atoms are there in 300 mole of h2o.
 Source: ask.learncbse.in
Source: ask.learncbse.in
Because one mole is specified as 6022 x 10 23 as well as there are 2 hydrogen per particle that would certainly indicate that there are specifically 12044 x 10 23 particles of hydrogen in one mole of water. The atom ratio and the mole ratio of the elements are identical. From the periodic table we see the atomic weight of hydrogen is. So there will be a total of 60210232121024 hydrogen atoms. One may also ask how many moles of hydrogen are in h2o.
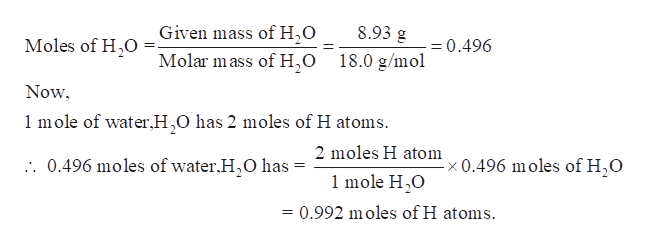 Source: bartleby.com
Source: bartleby.com
So 1 mole H2O 120441024 hydrogen atoms. Dual that for 2 moles as well as there are 24088 x 10 23 hydrogen atoms in one mole of water. Click to see full answer In respect to this how many hydrogen atoms are there in 300 mole of h2o. Thus 1 mole of water molecules will have 26022102312041024 2 6022 10 23 1204 10 24 hydrogen atoms. 2602310 231204610 23 atoms of hydrogen.
 Source: youtube.com
Source: youtube.com
Correct option is D The molar mass of water is 18 gmol. Answer 1 of 5. Mass of 1 Mole of Water Water H2O is made from 2 atoms of hydrogen and 1 atom of oxygen. Thus 1 mole of water molecules will have 26022102312041024 2 6022 10 23 1204 10 24 hydrogen atoms. In one mole of water there will exist approximately 6021023 water molecules.

Do this by adding up the mass of hydrogen atoms and oxygen atoms in a mole of water by looking up the atomic mass of hydrogen and oxygen on the periodic table. So a mole of water is 602 x 10 23 molecules of. From the periodic table we see the atomic weight of hydrogen is 10079 and the atomic weight of oxygen is 159994. Hence 12 g of water contains 0671204610 23602310 238010 23 hydrogen atoms. Answer 1 of 8.

120 g of water corresponds to 1812. Since water has a chemical formula of H2O there will be 2 moles of hydrogen in every mole of water. A mole of water molecules would be 2 moles of hydrogen atoms plus 1 mole of oxygen atoms. 1 molecule of water molecules has 2 atoms of hydrogen atoms. The chemical formula for aluminum hydroxide is AlOH3.

Since water has a chemical formula of H 2O there will be 2 moles of hydrogen in every mole of water. 2602310 231204610 23 atoms of hydrogen. Now number of moles of CH 4 in 52 1024 molecules is. So there will be 2 X 314 moles of H or 628 moles of hydrogen atoms remembering that a. From the periodic table we see the atomic weight of hydrogen is 10079 and the atomic weight of oxygen is 159994.
 Source: slideplayer.com
Source: slideplayer.com
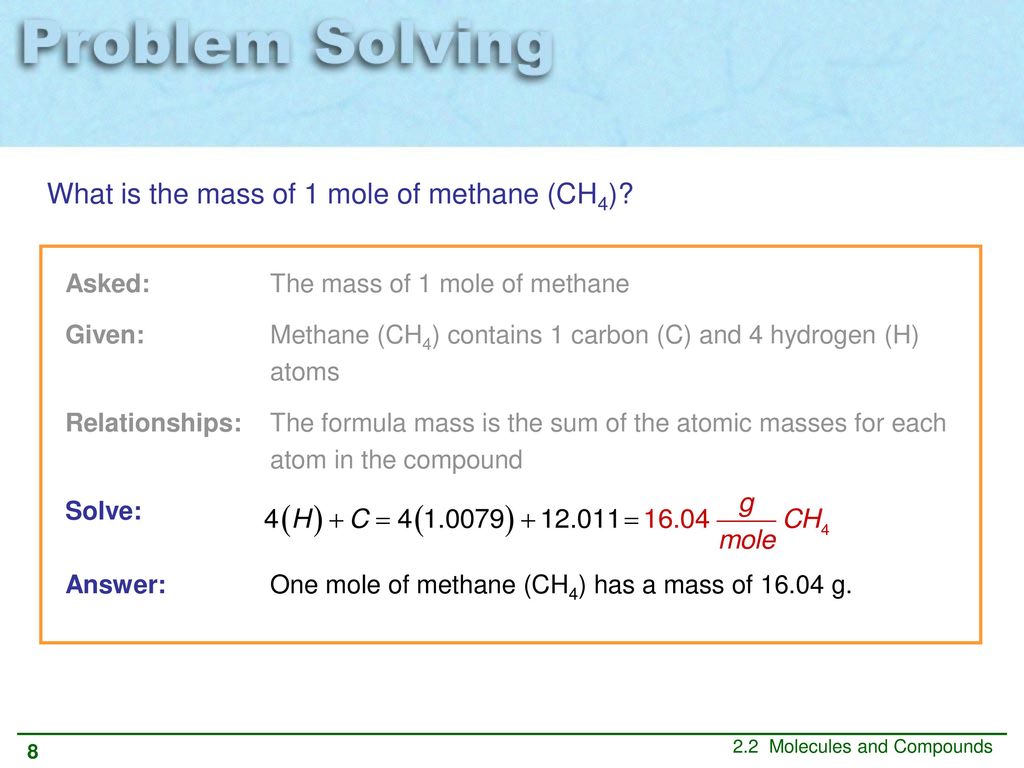
So 1 mole of CH 4 will have 4 mol atom of Hydrogen. The molecular formula of methane CH 4 suggests that one molecule of this compound consists of 4 atoms of H. Therefore 2 mole H2O will have 240881024 hydrogen atoms. From the periodic table we see the atomic weight of hydrogen is 10079 and the atomic weight of oxygen is 159994. So 3 moles of CO2 contains 3 x.
 Source: slidetodoc.com
Source: slidetodoc.com
Because one mole is specified as 6022 x 10 23 as well as there are 2 hydrogen per particle that would certainly indicate that there are specifically 12044 x 10 23 particles of hydrogen in one mole of water. Two Hydrogen atoms and one Oxygen atom combine into a water molecule. From the periodic table we see the atomic weight of hydrogen is. The one mole of any substance is equal to the 6023 times 1023 units of atoms molecules or ions. Similarly how many atoms are in 3 moles of co2.
 Source: courses.lumenlearning.com
Source: courses.lumenlearning.com
Because one mole is specified as 6022 x 10 23 as well as there are 2 hydrogen per particle that would certainly indicate that there are specifically 12044 x 10 23 particles of hydrogen in one mole of water. 1 water molecule 2 Hydrogen atoms 1 oxygen atom. The mass of oxygen equal to one mole of oxygen is 15998 grams and the mass of one mole of hydrogen is 1008 g. Since one mole is defined as 6022 x 1023 and there are two hydrogen per molecule that would mean that there are exactly 12044 x 1023 molecules of hydrogen in one mole of water. Each nitrate ion contains one nitrogen atom and three oxygen atoms.

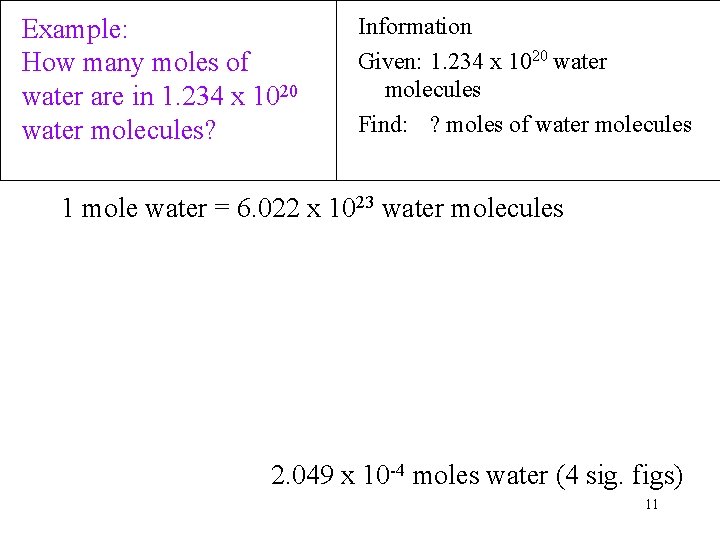
The mass of hydrogen is 1008 gmol and the mass of oxygen is 1600 gmol so the mass of a mole of water can be calculated as follows. Determine the molar mass of water. Now number of moles of CH 4 in 52 1024 molecules is. So 1 mole H2O 120441024 hydrogen atoms. In one mole of water there will exist approximately 602 1023 water molecules.

So there will be a total of 602 1023 2 12 1024 hydrogen atoms. Similarly how many hydrogen atoms are in a mole of water. 2602310 231204610 23 atoms of hydrogen. A mole of water molecules would be 2 moles of hydrogen atoms plus 1 mole of oxygen atoms. How many atoms are in H2SO4.
This site is an open community for users to submit their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site beneficial, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title how many hydrogen atoms in 1 mole of water by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






