Your How many grams per two moles are there in carbon images are available. How many grams per two moles are there in carbon are a topic that is being searched for and liked by netizens today. You can Get the How many grams per two moles are there in carbon files here. Download all royalty-free vectors.
If you’re searching for how many grams per two moles are there in carbon pictures information linked to the how many grams per two moles are there in carbon interest, you have pay a visit to the ideal site. Our website always gives you suggestions for viewing the highest quality video and image content, please kindly hunt and locate more informative video content and graphics that match your interests.
How Many Grams Per Two Moles Are There In Carbon. 6 moles Carbon to grams 720642 grams. 0112 moles CO2 x 6022x10 23 moleculemole x 2 O atomsmolecule. Then 239g44gmole 0543mole of C3H8 4. 6 moles Carbon Dioxide to grams 264057 grams.
 Moles From chemistry.wustl.edu
Moles From chemistry.wustl.edu
2 moles Carbon Dioxide to grams 88019 grams. The weight of CO2 is 44 grams per mole 1 x 12 gramsmole for the carbon and 2 x 16 gramsmole for the oxygen atoms. 2 moles Carbon to grams 240214 grams. 1 moles Carbon Dioxide to grams 440095 grams. 4 moles Carbon Monoxide to grams 1120404 grams. How many moles of carbon are in C6H12O6.
CO2 ratio one mole C3H8 produces three moles CO2 3.
1 moles Carbon to grams 120107 grams. Amazingly there are 602x1023 atoms in each of the samples above. 5 moles Co2 to grams 589332 grams. 6 moles Carbon to grams 720642 grams. One mole of any element or 1 g atom 6021023 atoms. Grams divided by the molar mass of C2H6 which is 180584gmol 722 moles of C2H6.
 Source: youtube.com
Source: youtube.com
4 moles Carbon Monoxide to grams 1120404 grams. Since 300 grams of CH4 is burned completely it is equal to 30016 grams per mole or 1875 moles. The molar mass is the amount of grams in one mole of a substance. 4 moles Carbon to grams 480428 grams. 1 moles Carbon Dioxide to grams 440095 grams.
 Source: youtube.com
Source: youtube.com
Burning one mole of octane 114 grams therefore would produce eight moles of. 5 moles Carbon to grams 600535 grams. 2 moles Carbon to grams 240214 grams. Therefore a mole of carbon atoms refers to a group of 6022 1023 carbon atoms. The molecular mass of H 2 O is 18gmol.

The amount of moles in a substance can be determined using that substances molar mass. One mole of CO2 is 44 grams and one mole of CH4 is 16 grams. The molar mass is the amount of grams in one mole of a substance. The molecular mass of H 2 O is 18gmol. 1 grams Carbon is equal to 0083259093974539 mole.

12 grams of carbon contains 1 mole. 6 moles Carbon Dioxide to grams 264057 grams. The result is the number of moles in your element or compound. 4 moles Carbon Dioxide to grams 176038 grams. One mole of CO2 is 44 grams and one mole of CH4 is 16 grams.

Grams divided by the molar mass of C2H6 which is 180584gmol 722 moles of C2H6. 2 moles Carbon Dioxide to grams 88019 grams. A sample of 12 grams of carbon is equal to one moleThe amount of moles in a substance can be determined using that substances molar mass. To compute for the number of grams of 1875 moles of CO2 44 grams per mole is multiplied by 1875 moles. 8 moles Co2 to grams 9429312 grams.
 Source: chemistry.wustl.edu
Source: chemistry.wustl.edu
5 moles Carbon to grams 600535 grams. 5 moles Carbon Monoxide to grams 1400505 grams. 10 moles Co2 to grams 1178664 grams. One mole of any element or 1 g atom 6021023 atoms. 86 g 44 gmole 01955 mole or about 02 moles.
 Source: opentextbc.ca
Source: opentextbc.ca
1200 g C-12 1 mol C-12 atoms 6022 1023 atoms The number of particles in 1 mole is called Avogadros Number 60221421 x 1023. 1200 g C-12 1 mol C-12 atoms 6022 1023 atoms The number of particles in 1 mole is called Avogadros Number 60221421 x 1023. A mole is a huge number used to measure atoms. 4 moles Carbon Dioxide to grams 176038 grams. The molecular mass of H 2 O is 18gmol.

6 moles Carbon Dioxide to grams 264057 grams. 1 moles Carbon Dioxide to grams 440095 grams. CO2 ratio one mole C3H8 produces three moles CO2 3. The amount of moles in a substance can be determined using that substances molar mass. 4 moles Co2 to grams 4714656 grams.

2 moles Co2 to grams 2357328 grams. Molecular weight of Carbon or mol. How many moles of carbon are in C6H12O6. 7 moles Carbon to grams 840749 grams. How many grams are in 2 moles of CO2.

The value of the mole is equal to the number of atoms in exactly 12 grams of pure carbon-12. 1 moles Carbon Dioxide to grams 440095 grams. 6 moles Carbon Monoxide to grams 1680606 grams. Just as its easier to measure intergalactic distances in light years rather than inches its easier to count atoms in moles than in billions or trillions. 4 moles Carbon to grams 480428 grams.
 Source: chemistry.wustl.edu
Source: chemistry.wustl.edu
6 moles Co2 to grams 7071984 grams. One mole of CO2 is 44 grams and one mole of CH4 is 16 grams. Grams divided by the molar mass of C2H6 which is 180584gmol 722 moles of C2H6. 5 moles Carbon to grams 600535 grams. Quick conversion chart of moles Carbon Dioxide to grams.
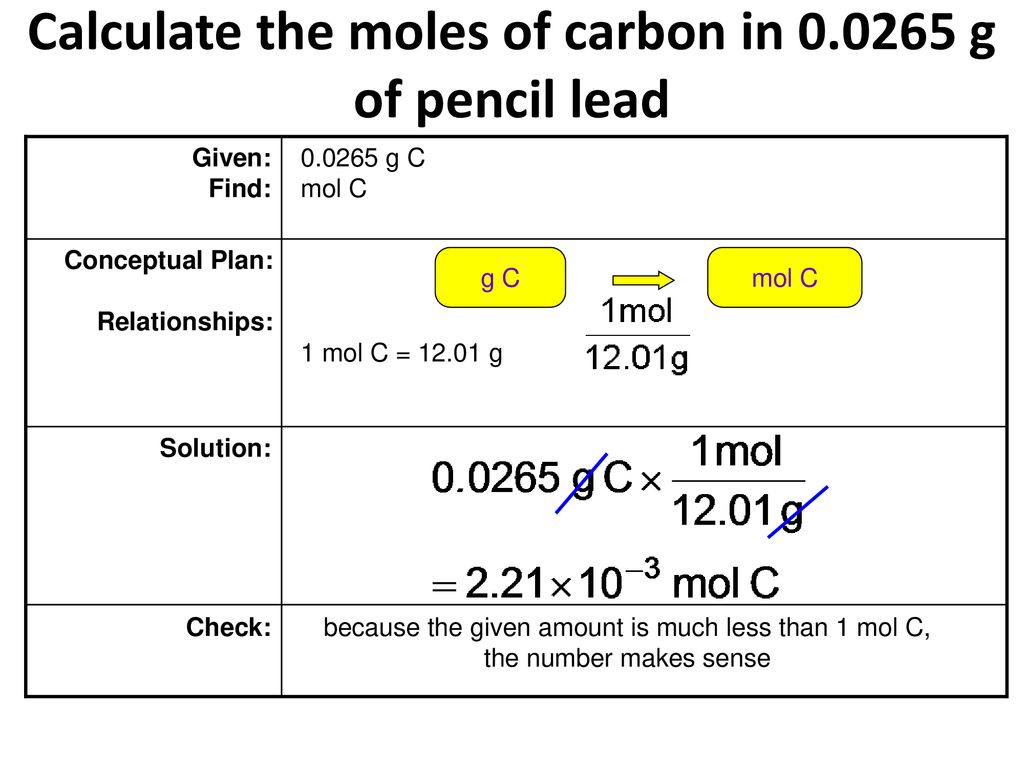 Source: geslab.net
Source: geslab.net
Atomic Weight of C 12. 8 g 844 211 moles. 3 moles Carbon Monoxide to grams 840303 grams. The molar mass is the amount of grams in one mole of a substance. Amazingly there are 602x1023 atoms in each of the samples above.
 Source: khanacademy.org
Source: khanacademy.org
6 moles Carbon to grams 720642 grams. 86 g 44 gmole 01955 mole or about 02 moles. 7 moles Carbon to grams 840749 grams. Now in mol of CO2 there are 6022x10 23 molecules and in each molecule there are 2 atoms of O. 250L x 1 mol224 L 0112 moles CO 2.
 Source: youtube.com
Source: youtube.com
4 moles Carbon Monoxide to grams 1120404 grams. Grams divided by the molar mass of C2H6 which is 180584gmol 722 moles of C2H6. 5 moles Carbon Dioxide to grams 2200475 grams. 1 mol of oxygen in contained in its molecular weight of 16 grams. 1200 g C-12 1 mol C-12 atoms 6022 1023 atoms The number of particles in 1 mole is called Avogadros Number 60221421 x 1023.
 Source: slideplayer.com
Source: slideplayer.com
1 mol of oxygen in contained in its molecular weight of 16 grams. How many grams are in 2 moles of CO2. 6 moles Co2 to grams 7071984 grams. This means that 1875 moles of CO2 is also produced. Burning one mole of octane 114 grams therefore would produce eight moles of.
This site is an open community for users to share their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site convienient, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title how many grams per two moles are there in carbon by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






