Your How many grams per five moles are there in hydrogen images are available in this site. How many grams per five moles are there in hydrogen are a topic that is being searched for and liked by netizens today. You can Find and Download the How many grams per five moles are there in hydrogen files here. Download all free vectors.
If you’re looking for how many grams per five moles are there in hydrogen images information linked to the how many grams per five moles are there in hydrogen topic, you have come to the right site. Our site always provides you with suggestions for seeing the highest quality video and picture content, please kindly surf and find more informative video content and images that match your interests.
How Many Grams Per Five Moles Are There In Hydrogen. How many individual HCl molecules are in a mass of 3646g of the gas. Do a quick conversion. 75 grams Hydrogen to mol 7440919 mol. 1 cubic foot of Hydrogen liquid weighs 436996 pounds lbs Hydrogen liquid weighs 007 gram per cubic centimeter or 70 kilogram per cubic meter ie.

There are 000896 moles of N. Calculate the moles present in 401 g Ca as follows. Avogadros number 6022xx1023mol-1. 2 moles Hydrogen Sulfide to grams 6816176 grams. If you have 1 mole the answer will be. 10 grams Hydrogen to mol 992123 mol.
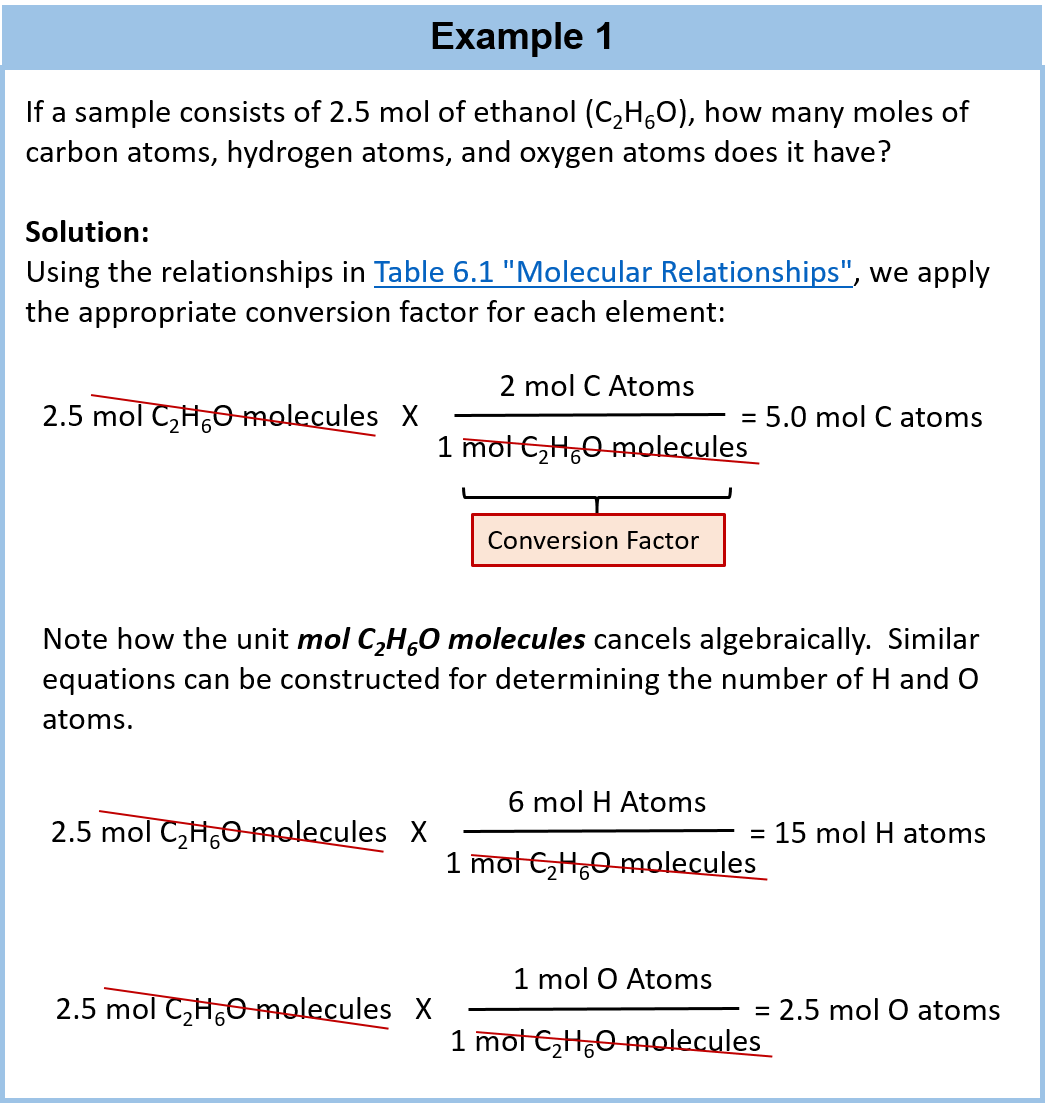
So 1 mole of sucrose contains 12 moles of carbon atoms 22 moles of hydrogen atoms and 11 moles of oxygen atoms.
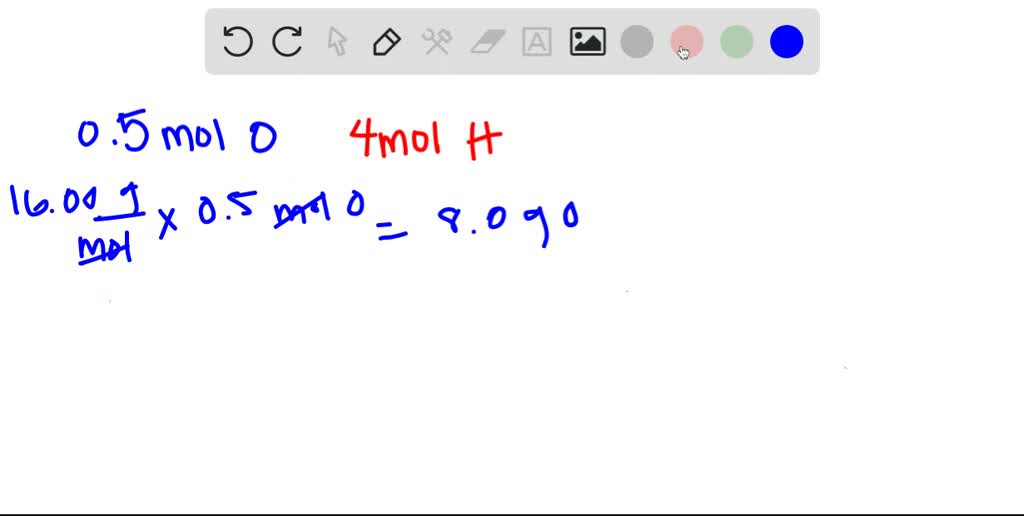
Hydrogen weighs 0000082 gram per cubic centimeter or 0082 kilogram per cubic meter ie. Three moles of hydrogen. At 0C 32F or 27315K at standard atmospheric pressure. How many grams are in 5 moles of hydrogen. 4 moles Hydrogen Peroxide to grams 13605872 grams. 10 moles Hydrogen to grams 100794 grams.
 Source: numerade.com
Source: numerade.com
Divide the mass of element in grams by its molar mass in gram per mole to obtain moles of the element. In Imperial or US customary measurement system the density is equal to 00051 pound per cubic foot lbft³ or 00000474 ounce per cubic inch ozinch³. 6 moles Hydrogen Sulfide to grams 20448528 grams. 5 grams Hydrogen to mol 496061 mol. Avogadros number 6022xx1023mol-1.
 Source: khanacademy.org
Source: khanacademy.org
Use hydrogens molar mass to determine how many grams would contain this many moles. Density of hydrogen is equal to 0082 kgm³. This is because Hydrogen atoms are smaller and lighter than Carbon atoms. How many individual HCl molecules are in a mass of 3646g of the gas. One mole of carbon C-12 has 12 grams of carbon.
 Source: clutchprep.com
Source: clutchprep.com
The entered volume of Hydrogen liquid in various units of volume. 10 moles Hydrogen to grams 100794 grams. 100 grams Hydrogen to mol. When you are talking about 1 mole of sucrose its the same as saying 1 mole of sucrose atoms so there are Avogadros number of atoms in one mole of sucrose or carbon or. 2 moles Hydrogen Sulfide to grams 6816176 grams.

While one mole of Hydrogen has 2 grams of Hydrogen. Divide the mass of element in grams by its molar mass in gram per mole to obtain moles of the element. 50 grams Hydrogen to mol 4960613 mol. How many individual HCl molecules are in a mass of 3646g of the gas. The value of the mole is equal to the number of atoms in exactly 12 grams of pure carbon-12.

How many moles are there in 25 grams to H2O. How many moles is this. 40 grams Hydrogen to mol 396849 mol. For example one mole ofatomic hydrogen has 1 gram. 151 x 1024.

10 grams of H2. How did I get that figure. 75 grams Hydrogen to mol 7440919 mol. Using molar mass we relate the number of atoms to mass. The molar mass of Ca is 4008 gmol.
 Source: bartleby.com
Source: bartleby.com
A person drinks 150 x 103 g of water H2O per day. How many moles are there in 392g of h2so4. So 1 mole of sucrose contains 12 moles of carbon atoms 22 moles of hydrogen atoms and 11 moles of oxygen atoms. How many atoms are there in 250 moles of H2O. There are two hydrogen atoms per water molecule.
 Source: youtube.com
Source: youtube.com
See also what are the characteristics of a species How many oxygen atoms are there in 3 molecules of H2O. Calculate the moles present in 401 g Ca as follows. 2 moles Hydrogen Peroxide to grams 6802936 grams. 1 000 000 000 000. In Imperial or US customary measurement system the density is equal to 00051 pound per cubic foot lbft³ or 00000474 ounce per cubic inch ozinch³.

How did I get that figure. At 0C 32F or 27315K at standard atmospheric pressure. How many moles are there in sucrose. Using molar mass we relate the number of atoms to mass. Use hydrogens molar mass to determine how many grams would contain this many moles.

The unit of mass is molar per mole or kilogram per mole. 5 moles Hydrogen to grams 50397 grams. 3 moles Hydrogen Sulfide to grams 10224264 grams. How many moles are there in 392g of h2so4. 5 grams Hydrogen to mol 496061 mol.

4 moles Hydrogen Peroxide to grams 13605872 grams. Density of hydrogen is equal to 0082 kgm³. If you look closely you will find that the molar mass is very close to the mass number of each atom. Using molar mass we relate the number of atoms to mass. 6 moles Hydrogen Peroxide to grams 20408808 grams.
 Source: wou.edu
Source: wou.edu
2 moles Hydrogen Peroxide to grams 6802936 grams. In Imperial or US customary measurement system the density is equal to 00051 pound per cubic foot lbft³ or 00000474 ounce per cubic inch ozinch³. 20 grams Hydrogen to mol 1984245 mol. Molar mass of Hydrogen Peroxide mol. 40 grams Hydrogen to mol 396849 mol.
 Source: clutchprep.com
Source: clutchprep.com
How many moles is this. The entered volume of Hydrogen liquid in various units of volume. 1200 g C-12 1 mol C-12 atoms 6022 1023 atoms The number of particles in 1 mole is called Avogadros Number 60221421 x 1023. 10 moles Hydrogen to grams 100794 grams. 1 grams Hydrogen Peroxide 0029399071224542 mole using the molecular weight calculator and the molar mass of H2O2.

How many moles are there in 392g of h2so4. The entered volume of Hydrogen liquid in various units of volume. A person drinks 150 x 103 g of water H2O per day. Divide the mass of element in grams by its molar mass in gram per mole to obtain moles of the element. Answer and Explanation.

How many atoms are there in 250 moles of H2O. 40 grams Hydrogen to mol 396849 mol. 1200 g C-12 1 mol C-12 atoms 6022 1023 atoms The number of particles in 1 mole is called Avogadros Number 60221421 x 1023. If you have 1 mole the answer will be. The entered volume of Hydrogen liquid in various units of volume.
This site is an open community for users to do submittion their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site helpful, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title how many grams per five moles are there in hydrogen by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






