Your How many grams of water are in one mole of water images are available in this site. How many grams of water are in one mole of water are a topic that is being searched for and liked by netizens today. You can Get the How many grams of water are in one mole of water files here. Get all free photos and vectors.
If you’re looking for how many grams of water are in one mole of water images information linked to the how many grams of water are in one mole of water topic, you have come to the right site. Our website always gives you suggestions for refferencing the highest quality video and image content, please kindly search and find more enlightening video content and graphics that match your interests.
How Many Grams Of Water Are In One Mole Of Water. 18 gram of water contains 1 mole of water. 1801528 grams of water per mole of water. A mole is a unit measuring the quantity of anything. Its molecular weight is 18 gmol.
 Lab How Big Is A Mole Intro To The Mole Concept Mole Concept Mole Physical Science From pinterest.com
Lab How Big Is A Mole Intro To The Mole Concept Mole Concept Mole Physical Science From pinterest.com
The mass of one mole of a substance is equal to that substances molecular weight. All we had to do is add up the grams that 2 moles of hydrogen plus 1 mole of oxygen weigh and we got our answer. From there you can go from moles to molecules using 6022 1023 molecules in a mole. 1 gram of water contains 1 18 mole of water. Similarly it is asked how many moles of water are there in one mole of hydrated salt. They do not affect the number of significant figures.
For example the mean molecular weight of water is 18015 atomic mass units amu so one mole of water weight 18015 grams.
The change of 1 g gram of water unit in a water measure measure equals into 100 ml milliliter of water as per the equivalent measure and for the same water measure type. The mass of one mole of a substance is equal to that substances molecular weight. This can also be written as 602210 23 mol-1The mass of one mole of a substance is equal to that substances molecular weight. Moles of water in one drop 005 grams x 1 mole18016 grams moles of water in one drop 0002775 moles. 1801528 grams of water per mole of water. So 200 g of water contains 200 18 moles of water.
 Source: toppr.com
Source: toppr.com
Was this answer helpful. 1 mole of water is equivalent to 18 g of water. Subsequently question is how many molecules of water are there in 36g of water. A T chart is useful for this kind of problem. A mole of water is about 18 grams.
 Source: youtube.com
Source: youtube.com
A T chart is useful for this kind of problem. Similarly it is asked how many moles of water are there in one mole of hydrated salt. 1 grams Water is equal to 0055508435061792 mole. The average mass of one mole of H2O is 1802 grams. A mole of water molecules would be 2 moles of hydrogen atoms plus 1 mole of oxygen atoms.
 Source: youtube.com
Source: youtube.com
Use this page to learn how to convert between grams Water and mole. A mole is a unit measuring the quantity of anything. For example the mean molecular weight of water is 18015 atomic mass units amu so one mole of water weight 18015 grams. 1 gram of water contains 1 18 mole of water. Fortunately this is another simple calculation.
 Source: pinterest.com
Source: pinterest.com
How many moles are in one gram of water. 1 mole is equal to 1 moles Water or 1801528 grams. 1111 moles of water. Subsequently question is how many molecules of water are there in 36g of water. 1018 moles 1018 6 1023 number of molecules 333 1023 molecules of water.
 Source: amburnclasses.org
Source: amburnclasses.org
The key to the conversion is realizing that 1 mole of any gas occupies 224L. Mass 1molmolar mass moles 100g a million00 mol18 g 56 moles word that the grams cancel molar mass is mass of one mole hence the 1molMW molecules a million00 mol602x1023molecules moles a million00x1024 molecules a million00mol 6021023 molecules a millionsixty seven mol lower back molecules as instruments cancel form of. Lets do the conversion now. Similarly it is asked how many moles of water are there in one mole of hydrated salt. So how many moles of water are in a glass of water.
 Source: youtube.com
Source: youtube.com
Each molecule of water has 3 atoms so the total number of atoms in 10g of water is 33331023. The SI base unit for amount of substance is the mole. Divide the mass of your anhydrous heated salt sample by the molar mass of the anhydrous compound to get the number of moles of compound present. This gives us a conversion factor of 1 mol224 L. Webew7 and 2 more users found this answer helpful.
 Source: pinterest.com
Source: pinterest.com
Therefore one mole of water weighs 180152 grams. How many moles are in one gram of water. The average mass of one mole of H2O is 1802 grams. A mole of water is about 18 grams. Use this page to learn how to convert between moles Water and gram.
 Source: slideplayer.com
Source: slideplayer.com
Since we only have 0001 grams that means that we have NA 000118 3351019 molecules of water. Considering an oxygen atom is sixteen times heavier than a hydrogen atom 1 mole of water has roughly 2 grams of hydrogen. The key to the conversion is realizing that 1 mole of any gas occupies 224L. The average mass of one mole of H2O is 1802 grams. Well we need to be specific about the glass of water.
 Source: slideplayer.com
Source: slideplayer.com
1018 moles 1018 6 1023 number of molecules 333 1023 molecules of water. Use this page to learn how to convert between grams Water and mole. Mass 1molmolar mass moles 100g a million00 mol18 g 56 moles word that the grams cancel molar mass is mass of one mole hence the 1molMW molecules a million00 mol602x1023molecules moles a million00x1024 molecules a million00mol 6021023 molecules a millionsixty seven mol lower back molecules as instruments cancel form of. 1 mole of water weighs 18g. 54 g H2O 1 mol H2O 180152 g H2O 6022 1023 molecules 1 mol H2O.

1111 moles of water. Considering an oxygen atom is sixteen times heavier than a hydrogen atom 1 mole of water has roughly 2 grams of hydrogen. Well we need to be specific about the glass of water. 1018 moles 1018 6 1023 number of molecules 333 1023 molecules of water. Therefore 1 mole of H2O got 18 grams.
 Source: youtube.com
Source: youtube.com
1 mole of hydrogen atoms got 1 gram so 2 moles of them got 2 grams. 1 grams Water is equal to 0055508435061792 mole. Therefore one mole of water weighs 180152 grams. For example the mean molecular weight of water is 18015 atomic mass units amu so one mole of water weight 18015 grams. The molar mass of water is 1802 gmol.
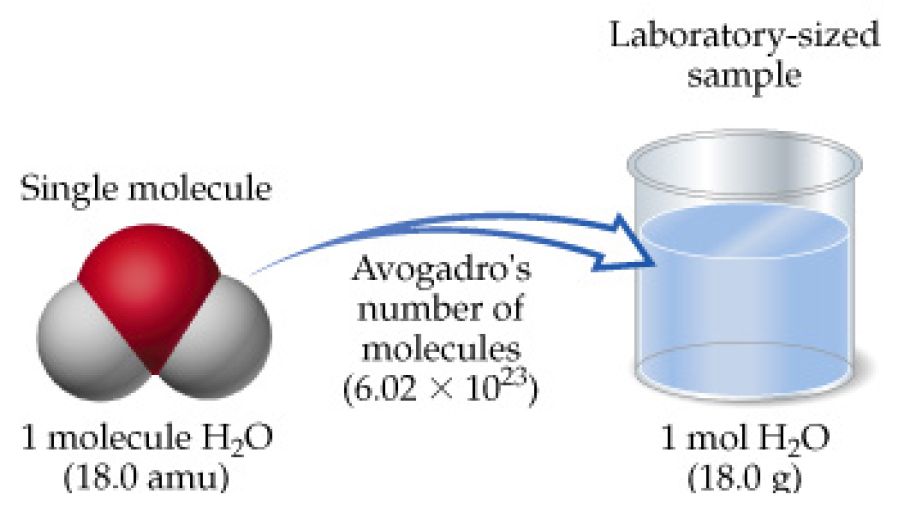 Source: socratic.org
Source: socratic.org
The SI base unit for amount of substance is the mole. 1 mole is equal to 1 moles Water or 1801528 grams. Unless you have a good sense of mass this value probably doesnt have much meaning to you. 54 g H2O 1 mol H2O 180152 g H2O 6022 1023 molecules 1 mol H2O. 1018 moles 1018 6 1023 number of molecules 333 1023 molecules of water.
 Source: youtube.com
Source: youtube.com
All we had to do is add up the grams that 2 moles of hydrogen plus 1 mole of oxygen weigh and we got our answer. Since we only have 0001 grams that means that we have NA 000118 3351019 molecules of water. The key to the conversion is realizing that 1 mole of any gas occupies 224L. The change of 1 g gram of water unit in a water measure measure equals into 100 ml milliliter of water as per the equivalent measure and for the same water measure type. 1 mole of hydrogen atoms got 1 gram so 2 moles of them got 2 grams.
 Source: pinterest.com
Source: pinterest.com
A mole of water is about 18 grams. All we had to do is add up the grams that 2 moles of hydrogen plus 1 mole of oxygen weigh and we got our answer. Therefore one mole of water weighs 180152 grams. The SI base unit for amount of substance is the mole. The SI base unit for amount of substance is the mole.
 Source: pinterest.com
Source: pinterest.com
Note that rounding errors may occur so always check the results. They do not affect the number of significant figures. 1 gram of water contains 1 18 mole of water. Was this answer helpful. 1 mole of hydrogen atoms got 1 gram so 2 moles of them got 2 grams.
This site is an open community for users to do sharing their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site good, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title how many grams of water are in one mole of water by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






