Your How many grams of nitrogen are in one mole of nitrogen gas images are available. How many grams of nitrogen are in one mole of nitrogen gas are a topic that is being searched for and liked by netizens today. You can Find and Download the How many grams of nitrogen are in one mole of nitrogen gas files here. Download all royalty-free images.
If you’re searching for how many grams of nitrogen are in one mole of nitrogen gas pictures information linked to the how many grams of nitrogen are in one mole of nitrogen gas keyword, you have visit the ideal site. Our website frequently provides you with suggestions for seeing the maximum quality video and image content, please kindly hunt and find more enlightening video articles and graphics that fit your interests.
How Many Grams Of Nitrogen Are In One Mole Of Nitrogen Gas. Click to see full answer. N2 g O2 g 2NO g Number of moles of O2 amount of O2molar mass of O2 221 g32 gmole 0691 moles According to the above balancedequation Considering N2 is excess 1 mole of O2 forms 2 moles of NO therefore Number of. Rounded to two sig figs the number of sig figs you gave for the temperature and volume of the gas the answer will be. Two atoms of nitrogen form the gaseous natural state of nitrogen.
 1 How Many Atoms Of Nitrogen Are Present Clutch Prep From clutchprep.com
1 How Many Atoms Of Nitrogen Are Present Clutch Prep From clutchprep.com
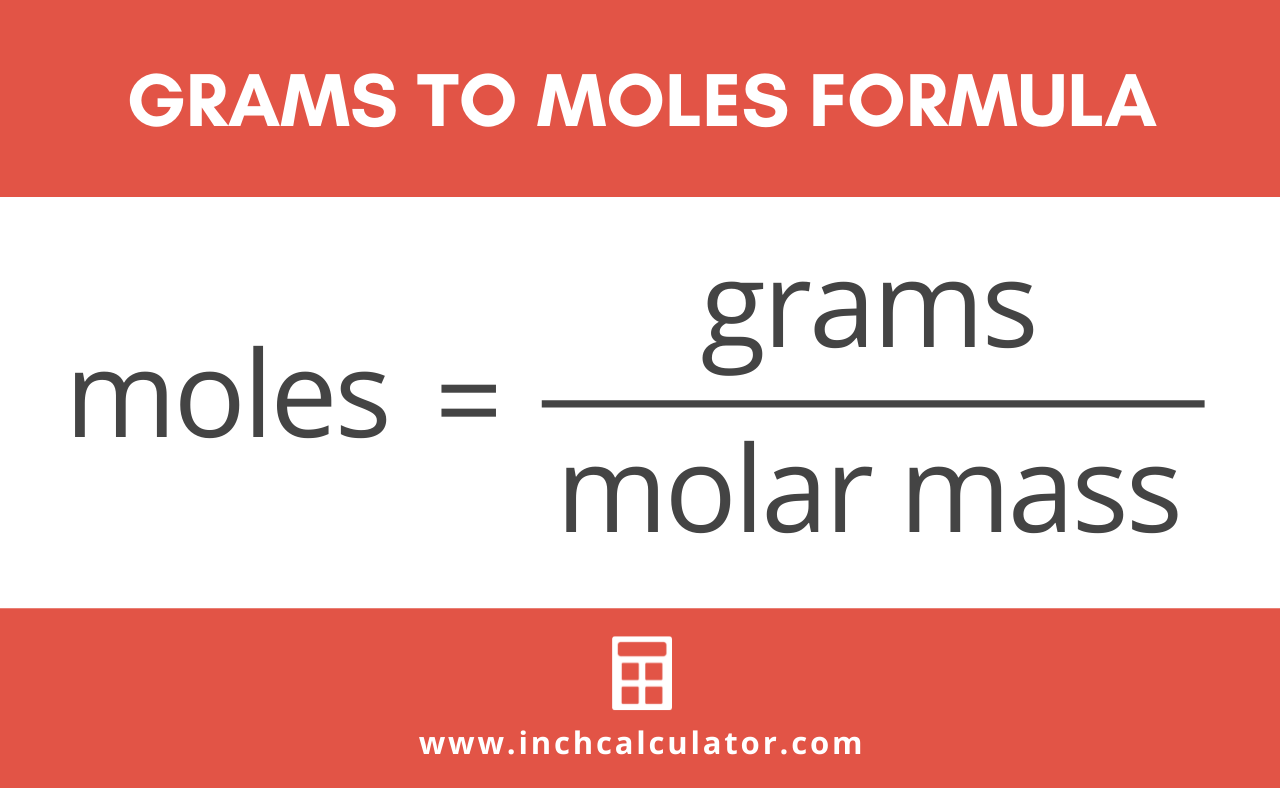
Therefore the mass of one mole of nitrogen gas N2 is 280 grams. Molecular weight of Nitrogen or mol The molecular formula for Nitrogen is N. 2 moles of nitrogen corresponds to 2 2 8 5 6 grams. But you could do it all in one step and you should still get the same answer. Also it is better for accounting reasons in order to find errors. The molecular formula for Nitrogen is N.
4 rows 1 000 000 000 000.
Therefore the mass of one mole of nitrogen gas N2 is 280 grams. So that means if you had 1 mole of nitrogen atoms it would weight 1400674 grams. So that means if you had 1 mole of nitrogen atoms it would weight 1400674 grams. Density of nitrogen gas is equal to 1251 kgm³. Nitrogen gas has consists of pairs of nitrogen atoms N2. And thus with respect to the nitrogen atom you gots two mole and with respect to the dinitrogen molecule you got a molar quantity.

4 rows 1 000 000 000 000. But since each individual molecule consists of 2 atoms of nitrogen the number of moles of nitrogen atoms will be twice that of nitrogen gas moleculesAlternatively you can express this as 2NA where NA is Avogadros constant. Like any elemental gas with any chemistry nitrogen occurs as the bimolecule N 2 ie. Now a mole is simply a very very large collection of particles. Molecular weight of Nitrogen or mol The molecular formula for Nitrogen is N.
 Source: slideplayer.com
Source: slideplayer.com
The mass of one mole molar mass of nitrogen gas N2 is 280 gmol May 22 2016. Royal Society Of Chemistry. 1 mole of H2O molecules has a mass of 1802 grams. Molar mass H2O 2atomic mass H 1atomic mass O 2100794 1159994 1802 1 H2O molecule has a mass of 1802 amu. So in one mole of nitrogen gas you have 60221023 molecules of nitrogen gas N2.
 Source: youtube.com
Source: youtube.com
If we divide both sides of the equation by the number 28 we get 28g28 202 x1023molecules28. According to the following reaction how many moles of nitrogen gas will be formed upon the complete reaction of 217 grams of ammonium nitrite. And thus with respect to the nitrogen atom you gots two mole and with respect to the dinitrogen molecule you got a molar quantity. We assume you are converting between grams Nitrogen and mole. So in one mole of nitrogen gas you have 60221023 molecules of nitrogen gas N2.

6 moles of hydrogen corresponds to 6 2 1 2 grams. Mass of one mole of nitrogen gas is 28 gram 142 mass of 05 mole of nitrogen is 14 grams. Ammonium nitrite aq nitrogen g water 1 moles nitrogen gas According to the. 4 rows 1 000 000 000 000. 2013moles 6501 g 1mole 13087 g.
 Source: youtube.com
Source: youtube.com
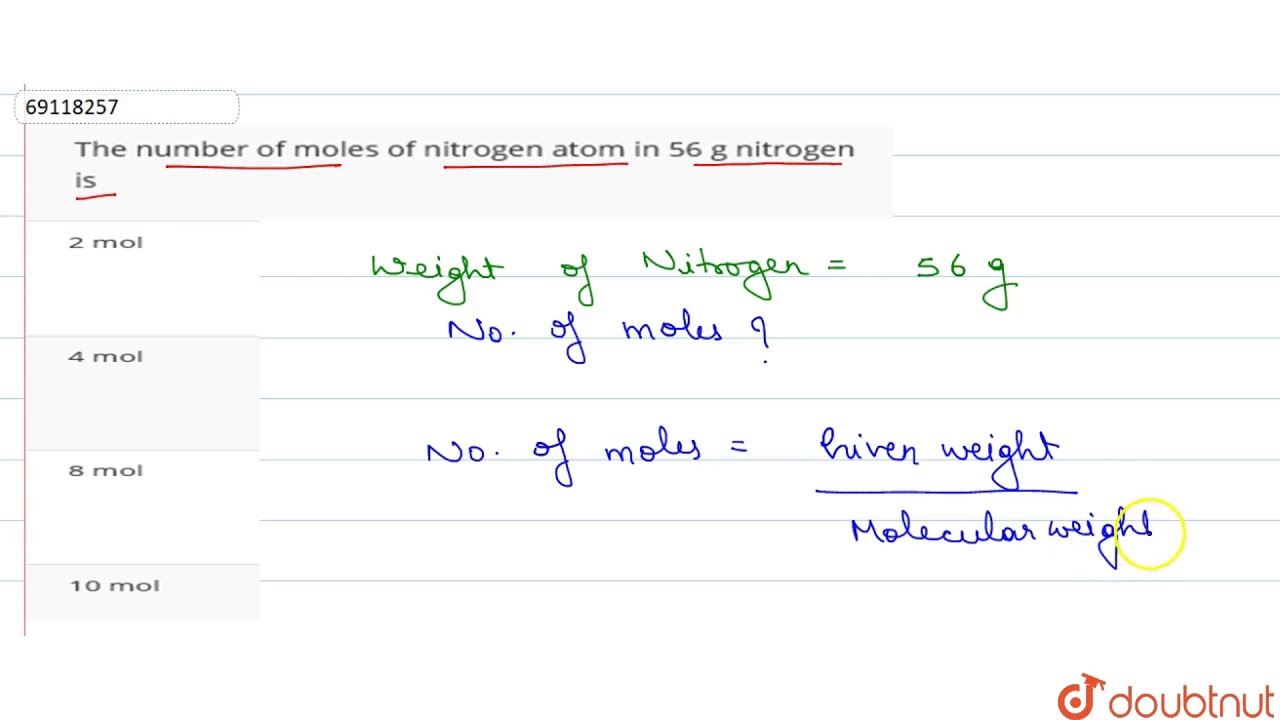
Nitrogen has an average atomic weight of 140067 so a mole of nitrogen gas is 280134 grams. So in one mole of nitrogen gas you have 60221023 molecules of nitrogen gas N2. So that means if you had 1 mole of nitrogen atoms it would weight 1400674 grams. To get the answer we have 28g 202 x1023 molecules. Alternatively you can express this as 2NA where NA is Avogadros constant.
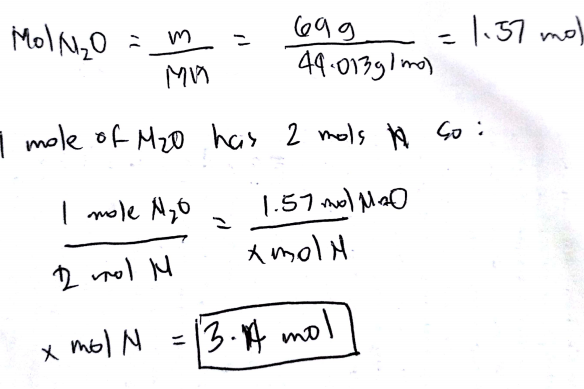 Source: clutchprep.com
Source: clutchprep.com
1062 grams N2 1 mole N22802 grams6022 X 10231 mole N21 mole N2 atoms6022 X 1023 03790 mole of gaseous nitrogen. And thus with respect to the nitrogen atom you gots two mole and with respect to the dinitrogen molecule you got a molar quantity. Therefore the mass of one mole of nitrogen gas N2 is 280 grams. Molar mass H2O 2atomic mass H 1atomic mass O 2100794 1159994 1802 1 H2O molecule has a mass of 1802 amu. Since nitrogens atomic mass is 140 there are 140 grams of nitrogen in one mole of nitrogen.
 Source: clutchprep.com
Source: clutchprep.com
The atomic mass of nitrogen is 14 grams per mole. Click to see full answer. MN aN 3 130 g. In order to have one mole of things lets say particles you need to have 6022 1023 particles this is known as Avogadros constant and acts as the definition of the mole. Therefore the mass of one mole of nitrogen gas N2 is 280 grams.
 Source: youtube.com
Source: youtube.com
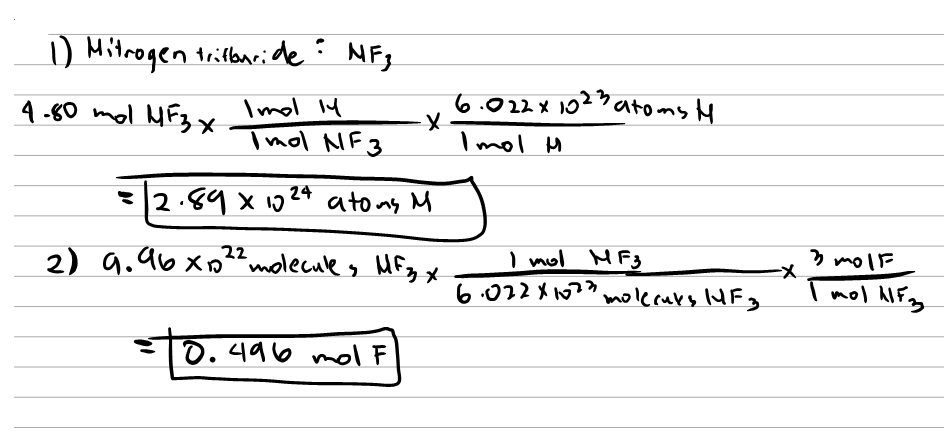
You know that one molecule of nitrogen gas N2 contains two atoms of nitrogen 2 N. But we want 1g how many molecules. But since each individual molecule consists of 2 atoms of nitrogen the number of moles of nitrogen atoms will be twice that of nitrogen gas molecules. To start you need know that all a mole means is you have 6022 x 10 23 atoms of that type. One mole of anything is defined as its molecular weight in grams.

View the full answer. Mass of one mole of nitrogen gas is 28 gram 142 mass of 05 mole of nitrogen is 14 grams. 2 moles of nitrogen corresponds to 2 2 8 5 6 grams. In order to have one mole of things lets say particles you need to have 6022 1023 particles this is known as Avogadros constant and acts as the definition of the mole. 4 rows 1 000 000 000 000.
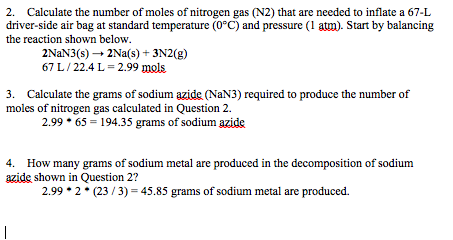 Source: chegg.com
Source: chegg.com
Nitrogen gas weighs 0001251 gram per cubic centimeter or 1251 kilogram per cubic meter ie. Solve any question of Some Basic Concepts of Chemistry with-. And thus with respect to the nitrogen atom you gots two mole and with respect to the dinitrogen molecule you got a molar quantity. Also it is better for accounting reasons in order to find errors. Ammonium nitrite aq nitrogen g water 1 moles nitrogen gas According to the.
 Source: youtube.com
Source: youtube.com
4 rows 1 000 000 000 000. Also it is better for accounting reasons in order to find errors. Now we have to get what fraction of a mole you have. So that means if you had 1 mole of nitrogen atoms it would weight 1400674 grams. We assume you are converting between grams Nitrogen and mole.
 Source: brainly.in
Source: brainly.in
The answer is 140067. Now we have to get what fraction of a mole you have. Since nitrogens atomic mass is 140 there are 140 grams of nitrogen in one mole of nitrogen. So that means if you had 1 mole of nitrogen atoms it would weight 1400674 grams. 6 moles of hydrogen corresponds to 6 2 1 2 grams.
 Source: clutchprep.com
Source: clutchprep.com
Also it is better for accounting reasons in order to find errors. For instance look at N Nitrogen you will see the atomic mass is 1400674 grams. Two atoms of nitrogen form the gaseous natural state of nitrogen. You can view more details on each measurement unit. Now we have to get what fraction of a mole you have.
 Source: toppr.com
Source: toppr.com
And thus with respect to the nitrogen atom you gots two mole and with respect to the dinitrogen molecule you got a molar quantity. Molar mass H2O 2atomic mass H 1atomic mass O 2100794 1159994 1802 1 H2O molecule has a mass of 1802 amu. To get the answer we have 28g 202 x1023 molecules. The SI base unit for amount of substance is the mole. Rounded to two sig figs the number of sig figs you gave for the temperature and volume of the gas the answer will be.
 Source: youtube.com
Source: youtube.com
Molar mass H2O 2atomic mass H 1atomic mass O 2100794 1159994 1802 1 H2O molecule has a mass of 1802 amu. Nitrogen has an average atomic weight of 140067 so a mole of nitrogen gas is 280134 grams. The SI base unit for amount of substance is the mole. MN aN 3 130 g. 2013moles 6501 g 1mole 13087 g.
This site is an open community for users to share their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site beneficial, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title how many grams of nitrogen are in one mole of nitrogen gas by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.