Your How many grams of nacl in one mole images are available. How many grams of nacl in one mole are a topic that is being searched for and liked by netizens today. You can Get the How many grams of nacl in one mole files here. Find and Download all free vectors.
If you’re searching for how many grams of nacl in one mole images information connected with to the how many grams of nacl in one mole interest, you have pay a visit to the ideal blog. Our website frequently gives you suggestions for downloading the maximum quality video and picture content, please kindly hunt and locate more enlightening video content and graphics that match your interests.
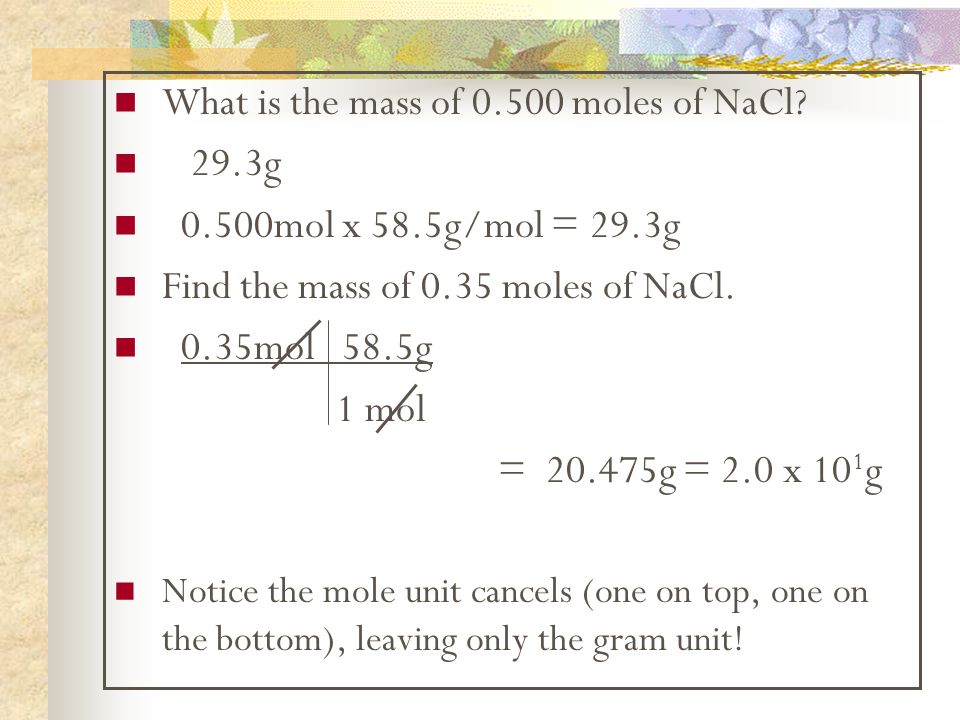
How Many Grams Of Nacl In One Mole. How many grams are in 1 mole of nacl. The mass in grams of a compound is equal to its molarity in moles multiply its molar mass. How do you calculate 1 mole of NaCl. The answer is 5844277.

To convert grams to moles you simple need to divide the mass of the sample by the molar mass of NaCl which can be found by adding the atomic masses of Na and Cl from periodic table and applying the unit grams. 1 mole is the same as 1 moles NaCl or 5844277 grams. So one mole of NaCl would weigh 584428 grams. A 002 M solution contains 002 moles per liter. Total molar mass 58442 gmol NaCl. As stated 1 mole of NaCl weighs 5844 g.
602 x 10 23.
What is the mole equation. This compound is also known as Sodium Chloride. This compound is also known as Sodium Chloride. Total molar mass 58442 gmol NaCl. 1 mole of NaCl 5844 grams the molar mass of Na which is 2299 gmol the molar mass of chlorine which is 3545 gmol 5844 gmol. One mol of NaCl 602 x1023 formulas has a mass of 5844 g.

This compound is also known as Sodium Chloride. To go from grams to moles divide the grams by the molar mass. The answer is 5844277. Rounding to one decimal place the mass of one mole of sodium Na is 230 grams and that of chlorine Cl is 355 grams so one mole of NaCl has a mass of 585 grams. The relation between molecular formula mass and molar mass.

In addition 2 moles of NaCl is formed when one mole of chlorine gas reacts. Total molar mass 58442 gmol NaCl. The answer is 0017110756386119. In our example 16. Avogadros number is a very important relationship to remember.
 Source: slideplayer.com
Source: slideplayer.com
1 grams NaCl is the same as 0017110756386119 mole. 1 grams NaCl is equal to 0017110756386119 mole. 1 mole is the same as 1 moles NaCl or 5844277 grams. Divide the mass of your anhydrous heated salt sample by the molar mass of the anhydrous compound to get the number of moles of compound present. 1946 moles would be 1946x584428 or 11373 grams.
 Source: slideserve.com
Source: slideserve.com
Then using stoichiometry you can find the grams of sodium. How many moles are in NaCl. 1946 moles would be 1946x584428 or 11373 grams. You can view more details on each measurement unit. 1 mole is the same as 1 moles NaCl or 5844277 grams.
 Source: slideplayer.com
Source: slideplayer.com
125 g NaCl 1 mole NaCl585 g 0214 moles. So one mole of NaCl would weigh 584428 grams. The answer is 0017110756386119. How many grams are in 1 mole of nacl. The SI base unit for amount of substance is the mole.
 Source: youtube.com
Source: youtube.com
You can view more details on each measurement unit. The SI base unit for amount of substance is the mole. Avogadros number is a very important relationship to remember. You can view more details on each measurement unit. You have 12 grams which equals 12585 021 moles.
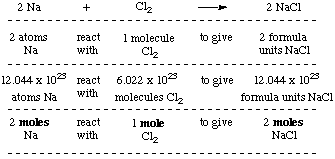 Source: chemiris.labs.brocku.ca
Source: chemiris.labs.brocku.ca
The molecular weight of sodium chloride NaCl is 5844 so one gram molecular weight 1 mole is 5844g. The molecular weight of sodium chloride NaCl is 5844 so one gram molecular weight 1 mole is 5844g. Rounding to one decimal place the mass of one mole of sodium Na is 230 grams and that of chlorine Cl is 355 grams so one mole of NaCl has a mass of 585 grams. Multiply this by Avogadros number to find that this amount of salt contains 451022 formula units of NaCl. However there are only 350 ml which is 035 liters.
 Source: youtube.com
Source: youtube.com
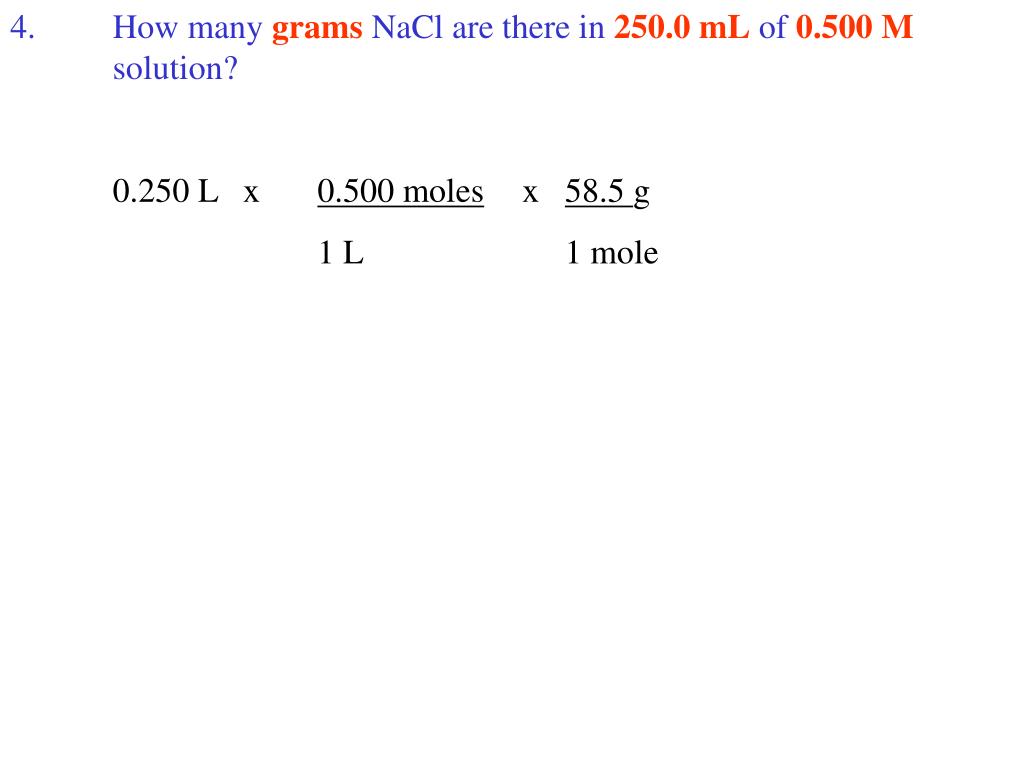
To obtain one mole of copper atoms 602 x 1023 atoms weigh out 6355 g copper. How many moles and how many grams of NaCl are present in 250 mL of a 05 M NaCl solution. We assume you might be changing between grams Sodium Chloride and mole. We assume you are converting between moles NaCl and gram. This compound is also known as Sodium Chloride.
 Source: slideplayer.com
Source: slideplayer.com
One mol of NaCl 602 x1023 formulas has a mass of 5844 g. Therefore there would be 035 x 11688 g in total 0409 g. So atomic mass Na 23 and atomic mass Cl 355. I dont have a periodic table with me so which you will would desire to seem up the molar mass. Multiply this by Avogadros number to find that this amount of salt contains 451022 formula units of NaCl.
 Source: slideplayer.com
Source: slideplayer.com
A 002 M solution contains 002 moles per liter. The molar mass of chlorine gas is 705 grams per mole while the molar mass of NaCl is 580 grams per mole. Molecular weight of NaCl or mol. Use this page to learn how to convert between moles NaCl and gram. 1 grams NaCl is equal to 0017110756386119 mole.
 Source: youtube.com
Source: youtube.com
125 g NaCl 1 mole NaCl585 g 0214 moles. You have 12 grams which equals 12585 021 moles. A 002 M solution contains 002 moles per liter. How do you calculate 1 mole of NaCl. 1 mole is the same as 1 moles NaCl or 5844277 grams.
 Source: slideplayer.com
Source: slideplayer.com
100 g NaCl 1 mol NaCl5844 g NaCl 1 mol Na 1 mol NaCl 2299 g of Na 1 mol Na which equals 39339435 g of Na. The molecular weight of sodium chloride NaCl is 5844 so one gram molecular weight 1 mole is 5844g. The molar mass of chlorine gas is 705 grams per mole while the molar mass of NaCl is 580 grams per mole. To convert between grams and moles you would use the substances molar mass. One formula unit of sodium chloride NaCl would weigh 5844 amu 2298977 amu for Na 35453 amu for Cl so a mole of sodium chloride would weigh 5844 grams.
 Source: clutchprep.com
Source: clutchprep.com
Divide the mass of your anhydrous heated salt sample by the molar mass of the anhydrous compound to get the number of moles of compound present. 1 mole 60221023 6022 10 23 atoms molecules protons etc. The mass in grams of a compound is equal to its molarity in moles multiply its molar mass. 125 g NaCl 1 mole NaCl585 g 0214 moles. Jul 18 2012 17.
 Source: youtube.com
Source: youtube.com
Molar mass of NaCl 5844277 gmol. 100 g NaCl 1 mol NaCl5844 g NaCl 1 mol Na 1 mol NaCl 2299 g of Na 1 mol Na which equals 39339435 g of Na. 584398 gmol 05 mol 29221 g 29 g rounded according to the least significant number Wiki User. As stated 1 mole of NaCl weighs 5844 g. The SI base unit for amount of substance is the mole.
 Source: slideplayer.com
Source: slideplayer.com
In addition 2 moles of NaCl is formed when one mole of chlorine gas reacts. Total molar mass 58442 gmol NaCl. Click hereto get an answer to your question How many moles and how many grams of NaCl are present in 250 mL of a 05 M NaCl solution. The answer is 0017110756386119. 600 g58443 gmol 1027 mol of NaCl.
This site is an open community for users to submit their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site convienient, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title how many grams of nacl in one mole by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






