Your How many grams of nacl equal one mole images are available. How many grams of nacl equal one mole are a topic that is being searched for and liked by netizens now. You can Get the How many grams of nacl equal one mole files here. Download all royalty-free images.
If you’re searching for how many grams of nacl equal one mole images information connected with to the how many grams of nacl equal one mole topic, you have come to the right site. Our site always provides you with suggestions for seeing the maximum quality video and image content, please kindly hunt and locate more informative video content and images that match your interests.
How Many Grams Of Nacl Equal One Mole. The SI base unit for quantity of substance is the mole. 1 mole of NaCl 23355585g. 0325 171. Feb 25 2020 Rounding to one decimal place the mass of one mole of sodium Na is 230 grams and that of chlorine Cl is 355 grams so one mole of NaCl has a mass of 585 grams.

One molecule of water H2O would weigh 1802 amu 2100797 amu for H 159994 amu for O and a mole of water molecules would weigh 1802 grams. What is the mole equation. A a mNaCl 1 mole 5844247 g a a. Feb 25 2020 Rounding to one decimal place the mass of one mole of sodium Na is 230 grams and that of chlorine Cl is 355 grams so one mole of NaCl has a mass of 585 grams. 1 mole is equal to 1 moles NaCl or 5844277 grams. Use this page to learn how to convert between moles NaCl and gram.
One molecule of water H2O would weigh 1802 amu 2100797 amu for H 159994 amu for O and a mole of water molecules would weigh 1802 grams.
1 mole is equal to 1 moles NaCl or 5844277 grams. Molecular weight of NaClO3 or grams. 1 mole is equal to 1 moles 2 NaCl or 6044277 grams. People also asked How many moles are in 10 kilograms of NaCl. Therefore 585 gm of sodium chloride is equal to one mole of sodium chloride. 1 grams NaCl is equal to 0017110756386119 mole.
 Source: slideplayer.com
Source: slideplayer.com
1 mole 60221023 6022 10 23 atoms molecules protons etc. This compound is also known as Sodium Chloride. The number of moles formula is given by. Determine the number of moles in 0325g of barium hydroxide. 2298977 35453 Percent composition by element.
 Source: slideplayer.com
Source: slideplayer.com
Use this page to learn how to convert between moles NaCl and gram. M M NaCl 5844247 g mol1. Rounding to one decimal place the mass of one mole of sodium Na is 230 grams and that of chlorine Cl is 355 grams so one mole of NaCl has a mass of 585 grams. 1 mole is equal to 1 moles 2 NaCl or 6044277 grams. The answer is 0010502988100114We assume you are converting between moles MgCl2 and gram.
 Source: slideplayer.com
Source: slideplayer.com
Therefore 585 gm of sodium chloride is equal to one mole of sodium chloride. 1 grams NaCl is equal to 0017110756386119 mole. A a mNaCl 1 mole 5844247 g a a. The answer is 0017110756386119. One formula unit of sodium chloride NaCl would weigh 5844 amu 2298977 amu for Na 35453 amu for Cl so a mole of sodium chloride would weigh 5844 grams.
 Source: youtube.com
Source: youtube.com
1 mole of NaCl is 5844 g 200 g NaCl 1 mol NaCl5844 g NaCl 3422 mol NaCl There are about 34 moles in 200 grams of NaCl. 1 mole is equal to 1 moles NaCl or 5844277 grams. How many grams are in an H. You can view more details on each measurement unit. Note that rounding errors may occur so always check the results.
 Source: slideplayer.com
Source: slideplayer.com
1 mole is equal to 1 moles NaClO3 or 10644097 grams. Molecular weight of NaClO3 or grams. 1 mole of NaCl 23355585g. The SI base unit for quantity of substance is the mole. The molecular weight of NaCl is 5844 gramsmole.
 Source: clutchprep.com
Source: clutchprep.com
Note that rounding errors may occur so always check the results. In this video well learn to convert moles of NaCl to grams. Avogadros number is a very important relationship to remember. The SI base unit for amount of substance is the mole. 1 mole is equal to 1 moles NaCl or 5844277 grams.
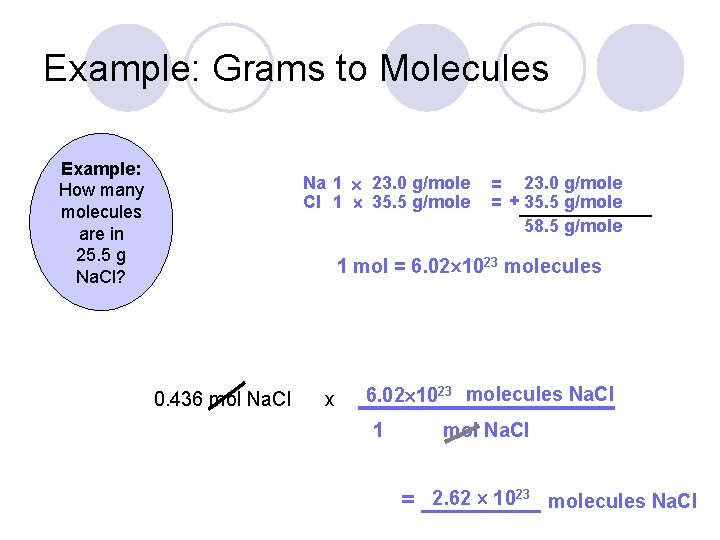 Source: slidetodoc.com
Source: slidetodoc.com
You have 12 grams which equals 12585 021 moles. Note that rounding errors may occur so always check the results. One molecule of water H2O would weigh 1802 amu 2100797 amu for H 159994 amu for O and a mole of water molecules would weigh 1802 grams. Half 2925 gm. 1 mole is equal to 1 moles NaClO3 or 10644097 grams.

0325 171. Given values are Number of moles formula is expressed by. Therefore 585 gm of sodium chloride is equal to one mole of sodium chloride. Note that rounding errors may occur so always check the results. 05 mole of Na 05 mole of Cholride is equal to one Gross mole.
 Source: youtube.com
Source: youtube.com
05 mole of Na 05 mole of Cholride is equal to one Gross mole. To know the molecular weight of the compound we have to add the molecular weights of the individual atoms present in the molecular formula of the compound. Number of moles Mass of substance Mass of one mole. Feb 25 2020 Rounding to one decimal place the mass of one mole of sodium Na is 230 grams and that of chlorine Cl is 355 grams so one mole of NaCl has a mass of 585 grams. 1 mole is the same as 1 moles NaCl or 5844277 grams.

This compound is also known as Sodium Chloride. Molecular weight of H or grams The SI base unit for amount of substance is the mole. This compound is also known as Sodium Chloride. 1 mole of NaCl is 5844 g 200 g NaCl 1 mol NaCl5844 g NaCl 3422 mol NaCl There are about 34 moles in 200 grams of NaCl. The answer is 0010502988100114We assume you are converting between moles MgCl2 and gram.

Given values are Number of moles formula is expressed by. The answer is 0010502988100114We assume you are converting between moles MgCl2 and gram. Now look into as a play game. One formula unit of sodium chloride NaCl would weigh 5844 amu 2298977 amu for Na 35453 amu for Cl so a mole of sodium chloride would weigh 5844 grams. 1 mole of NaCl is 5844 g 200 g NaCl 1 mol NaCl5844 g NaCl 3422 mol NaCl There are about 34 moles in 200 grams of NaCl.
 Source: youtube.com
Source: youtube.com
2925 not equal to 31737 gm. How many grams are in an H. The answer is 0010502988100114We assume you are converting between moles MgCl2 and gram. How do you calculate 1 mole of NaCl. 1 grams NaCl is equal to 0017110756386119 mole.
 Source: chemiris.labs.brocku.ca
Source: chemiris.labs.brocku.ca
N Total mass Molar mass. 1 mole is equal to 1 moles NaCl or 5844277 grams. Number of moles Mass of substance Mass of one mole. This means that the mass of one mole of sodium chloride is equal to. One mole is defined as the amount of substance containing as many elementary entities atoms molecules ions electrons radicals etc as there are atoms in 12 grams of carbon-12 6023 x 1023.
 Source: slideplayer.com
Source: slideplayer.com
Molar mass of NaCl 5844277 gmol. The answer is 0017110756386119. Use this page to learn how to convert between grams NaCl and mole. N Total mass Molar mass. The SI base unit for quantity of substance is the mole.
 Source: slideplayer.com
Source: slideplayer.com
Note that rounding errors may occur so always check the results. One mole is defined as the amount of substance containing as many elementary entities atoms molecules ions electrons radicals etc as there are atoms in 12 grams of carbon-12 6023 x 1023. NaCl molecular weight. 1 mole is equal to 1 moles MgCl2 or 95211 grams. This means that the mass of one mole of sodium chloride is equal to.
This site is an open community for users to do sharing their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site serviceableness, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title how many grams of nacl equal one mole by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






