Your How many grams of carbon are in 1 mole of glucose c6h12o6 images are available. How many grams of carbon are in 1 mole of glucose c6h12o6 are a topic that is being searched for and liked by netizens today. You can Get the How many grams of carbon are in 1 mole of glucose c6h12o6 files here. Download all royalty-free images.
If you’re searching for how many grams of carbon are in 1 mole of glucose c6h12o6 images information connected with to the how many grams of carbon are in 1 mole of glucose c6h12o6 topic, you have come to the ideal blog. Our site always provides you with hints for seeing the maximum quality video and image content, please kindly search and locate more informative video articles and graphics that fit your interests.
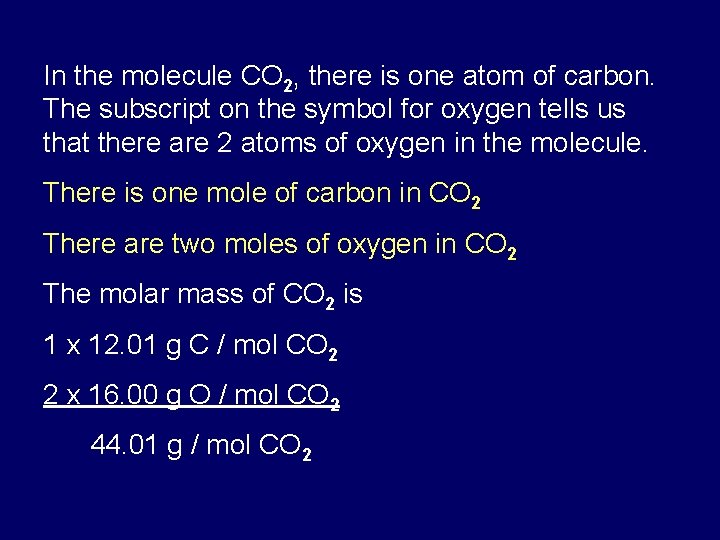
How Many Grams Of Carbon Are In 1 Mole Of Glucose C6h12o6. Since there are 6 atoms of carbon we take the mass of carbon 120107 u and multiply it by 6. Molar mass of oxygen O16gmol1. To get a single glucose molecule C6H12O6 we need 6 carbon atoms 12 hydrogen atoms and finally 6 oxygen atoms. A Mass of 1 mol of glucose C6H12O6M 6 x 1201 gmol C 12 x 1008 gmol H 6 x 1600 gmol O 18016 g C6H12O6Mass percent of C H and OMass C 6 mol C x 1201 gmol C x 100 4000 mass C18016 g C6H.
 Quick Review The Mole A Very Large Counting From slidetodoc.com
Quick Review The Mole A Very Large Counting From slidetodoc.com
01 X Avagadros number 602 x 10 exp24 x 6number of carbons in each 01 mole 360 x 10 exp23. What mass of water must be added to 05 mol of glucose to make a solution of 25 percent by massThe molar mass of glucose is 180 gmol. A Mass of 1 mol of glucose C6H12O6M 6 x 1201 gmol C 12 x 1008 gmol H 6 x 1600 gmol O 18016 g C6H12O6Mass percent of C H and OMass C 6 mol C x 1201 gmol C x 100 4000 mass C18016 g C6H. You can view more details on each measurement unit. This preview shows page 14 - 20 out of 25 pages. Molecular weight of C6H12O6 or mol.
Molar mass of Carbon C12gmol1.
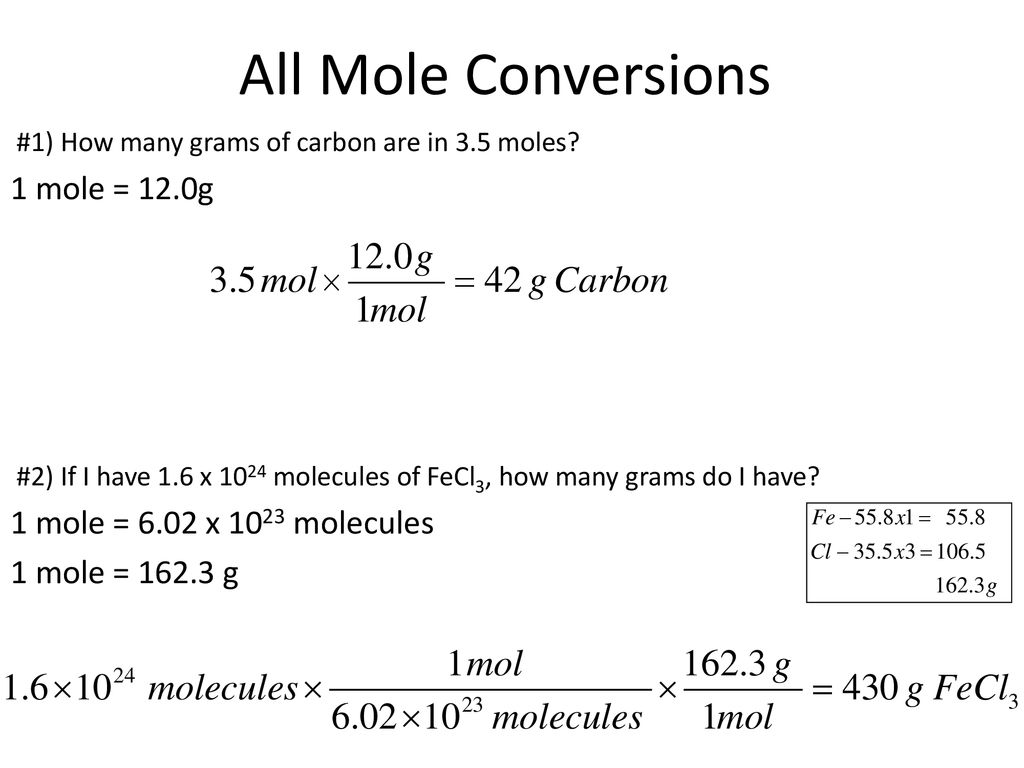
You can view more details on each measurement unit. Therefore your 90-g sample will contain. 132 x 12 x 602 x1023 954 x 1024 hydrogen atoms in 132 moles of glucose. 4 rows Molar mass of C6H12O6 18015588 gmol. How many moles of carbon are in C6H12O6. BHow many grams of carbon are in 1655 g of glucose.
 Source: diabetestalk.net
Source: diabetestalk.net
To get a single glucose molecule C6H12O6 we need 6 carbon atoms 12 hydrogen atoms and finally 6 oxygen atoms. Mass of 1 mole of glucose C6H12O6 6 1201 12 101 6 1600 g 18018 g using atomic weight data to 2 decimals 1 mole of carbon atoms weighs 1201 g and there are 6 moles of C atoms in 1 mole of glucose so the mass of carbon in 1 mole of glucose 6 1201 g 7206 g. Therefore your 90-g sample will contain. Given mass of glucose 300g. This compound is also known as Glucose or Fructose or Galactose.
 Source: slideplayer.com
Source: slideplayer.com
When molecular mass of an atom is expressed in grams it is called one mole. All of this weighs So the molecular weight or weight of a mole of sugar is 180g. Answer 1 of 1. The answer is 18015588. Up to 20 cash back One type of anaerobic respiration converts glucose C6H12O6 to ethanol C2H5OH and carbon dioxide.
 Source: numerade.com
Source: numerade.com
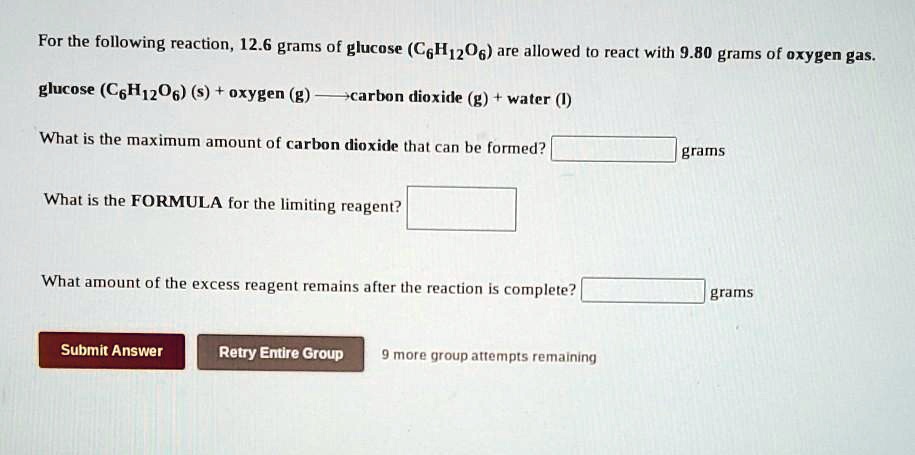
There are 602 x 1023 particles in a mole so 180 g of glucose must contain 6 x 602 x 1023 carbon atoms. A solution contains 180g of glucose C6H12O6 and 162 g. The given glucose sample mass is. This equals 361 x 1024 carbon atoms. What mass of water must be added to 05 mol of glucose to make a solution of 25 percent by massThe molar mass of glucose is 180 gmol.
 Source: youtube.com
Source: youtube.com
Molar mass of Hydrogen H1gmol1. From that we get 720642 and multiply it by the amount of moles which is 195. This compound is also known as Glucose or Fructose or. 90g 1 mole C6H12O6 180156g 04996 moles C5H12O6. There are 602 x 1023 particles in a mole so 180 g of glucose must contain 6 x 602 x 1023 carbon atoms.
 Source: studylib.net
Source: studylib.net
How many moles of carbon are in C6H12O6. C6H12O6 C 6 H 12 O 6. A Mass of 1 mol of glucose C6H12O6M 6 x 1201 gmol C 12 x 1008 gmol H 6 x 1600 gmol O 18016 g C6H12O6Mass percent of C H and OMass C 6 mol C x 1201 gmol C x 100 4000 mass C18016 g C6H. This compound is also known as Glucose or Fructose or Galactose. Glucose has a molar mass of 180156 gmol which means that one mole of glucose molecules has a mass of 180156 g.
 Source: slideplayer.com
Source: slideplayer.com
A Mass of 1 mol of glucose C6H12O6M 6 x 1201 gmol C 12 x 1008 gmol H 6 x 1600 gmol O 18016 g C6H12O6Mass percent of C H and OMass C 6 mol C x 1201 gmol C x 100 4000 mass C18016 g C6H. How many grams are in one mole of C6H12O6. There are 602 x 1023 particles in a mole so 180 g of glucose must contain 6 x 602 x 1023 carbon atoms. 560 g C6H12O6 1 mole C6H12O6180156 g6 moles C1 moleC6H12O66022 X 10231 mole C 112 X 1023 atoms of carbon. This compound is also known as Glucose or Fructose or Galactose.
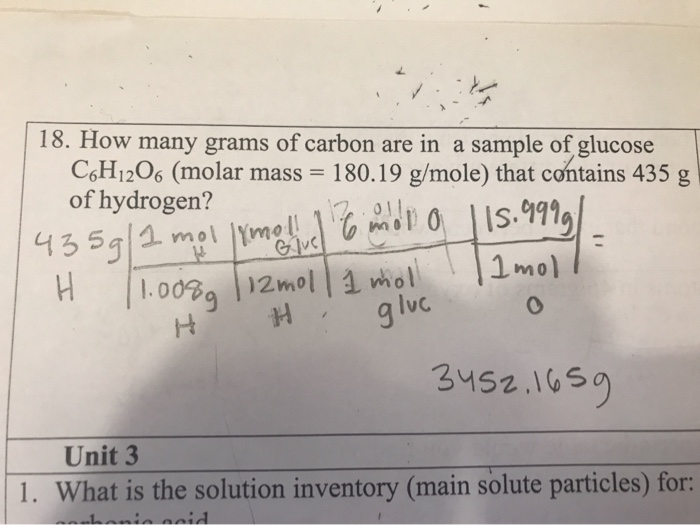 Source: chegg.com
Source: chegg.com
This compound is also known as Glucose or Fructose or Galactose. Answer 1 of 1. How many moles of carbon are in one mole of C6H12O6. A Mass of 1 mol of glucose C6H12O6M 6 x 1201 gmol C 12 x 1008 gmol H 6 x 1600 gmol O 18016 g C6H12O6Mass percent of C H and OMass C 6 mol C x 1201 gmol C x 100 4000 mass C18016 g C6H. Mass of 1 mole of glucose C6H12O6 6 1201 12 101 6 1600 g 18018 g using atomic weight data to 2 decimals 1 mole of carbon atoms weighs 1201 g and there are 6 moles of C atoms in 1 mole of glucose so the mass of carbon in 1 mole of glucose 6 1201 g 7206 g.
 Source: in.pinterest.com
Source: in.pinterest.com
Mass of 1 mole of glucose C6H12O6 6 1201 12 101 6 1600 g 18018 g using atomic weight data to 2 decimals 1 mole of carbon atoms weighs 1201 g and there are 6 moles of C atoms in 1 mole of glucose so the mass of carbon in 1 mole of glucose 6 1201 g 7206 g. Mglucose 150 g m g l u c o s e 150 g. When molecular mass of an atom is expressed in grams it is called one mole. This preview shows page 14 - 20 out of 25 pages. C6H12O6 C 6 H 12 O 6.
 Source: in.pinterest.com
Source: in.pinterest.com
There are 6 carbon atoms in a glucose molecule so that timessix would give you a. Mglucose 150 g m g l u c o s e 150 g. Glucose has a molar mass of 180156 gmol which means that one mole of glucose molecules has a mass of 180156 g. A Mass of 1 mol of glucose C6H12O6M 6 x 1201 gmol C 12 x 1008 gmol H 6 x 1600 gmol O 18016 g C6H12O6Mass percent of C H and OMass C 6 mol C x 1201 gmol C x 100 4000 mass C18016 g C6H. You can view more details on each measurement unit.
 Source: slidetodoc.com
Source: slidetodoc.com
Chemistry the density at 20 degrees celcius of a 258m solution of glucose in water is 10173 gml and the molar mass of glucose is 1802 gmol. If the molecular weight of glucose is 180 gramsmol and molar mass of ethanol is 46 how many grams of carbon dioxide are produced when 1 mol of glucose is digested via respiration. Mass of 1 mole of glucose C6H12O6 6 1201 12 101 6 1600 g 18018 g using atomic weight data to 2 decimals 1 mole of carbon atoms weighs 1201 g and there are 6 moles of C atoms in 1 mole of glucose so the mass of carbon in 1 mole of glucose 6 1201 g 7206 g. A mole of C12H22O11 would have a mass of 342299 grams. When molecular mass of an atom is expressed in grams it is called one mole.
 Source: slideplayer.com
Source: slideplayer.com
1 molecule of glucose contains 6 atoms of C 12 atoms of H and 6 atoms of O 1 mole of glucose contains 6 moles of C atoms 12 moles of H atoms and 6 moles of O atoms. Now we can calculate the number of moles in given mass of glucose using the below formula. BHow many grams of carbon are in 1655 g of glucose. Glucose is C6H12O6 with a molar mass of approximately 180 grams so 18 grams is 01 moles. You can view more details on each measurement unit.
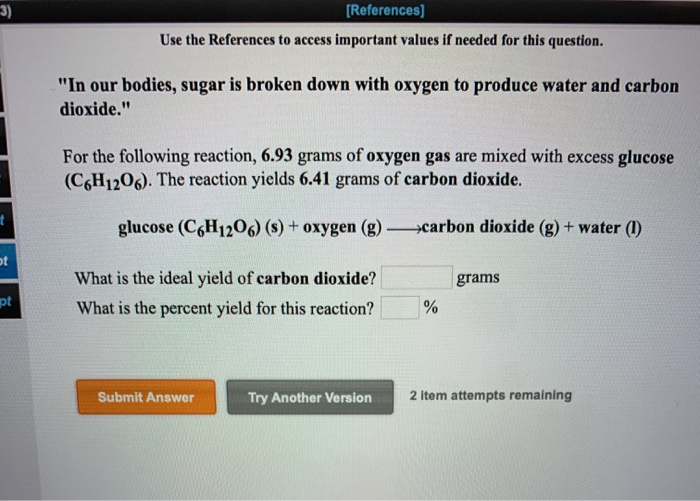 Source: chegg.com
Source: chegg.com
Mglucose 150 g m g l u c o s e 150 g. A solution contains 180g of glucose C6H12O6 and 162 g. This preview shows page 14 - 20 out of 25 pages. Glucose is a covalent compound with the given molecular formula. How many moles of carbon are in one mole of C6H12O6.
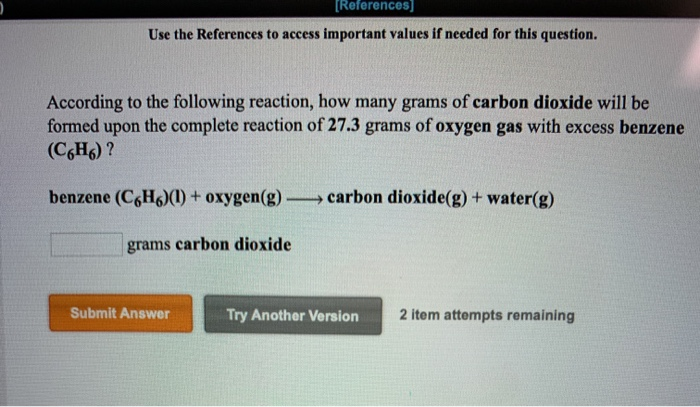 Source: chegg.com
Source: chegg.com
This preview shows page 14 - 20 out of 25 pages. BHow many grams of carbon are in 1655 g of glucose. This preview shows page 14 - 20 out of 25 pages. All of this weighs So the molecular weight or weight of a mole of sugar is 180g. Mglucose 150 g m g l u c o s e 150 g.

From that we get 720642 and multiply it by the amount of moles which is 195. 4 rows Molar mass of C6H12O6 18015588 gmol. According to the Avogadros number one mole ofa substance contain 6022x 1023 moleculesOne mole of glucose 6022x 1023 moleculesthree moles of glucose 36022x 1023 moleculesIn a molecule of glucose there are 24 atoms. C6H12O6 C 6 H 12 O 6. This compound is also known as Glucose or Fructose or Galactose.
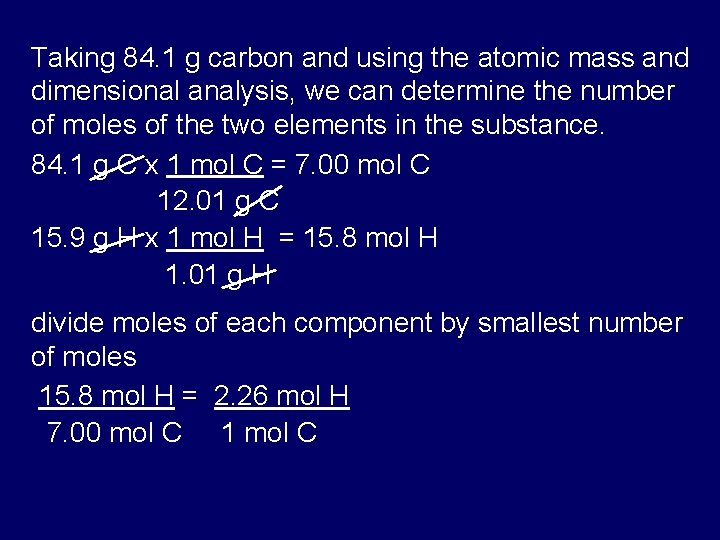 Source: slidetodoc.com
Source: slidetodoc.com
Given mass of glucose 300g. Molecular weight of C6H12O6 or mol. A Mass of 1 mol of glucose C6H12O6M 6 x 1201 gmol C 12 x 1008 gmol H 6 x 1600 gmol O 18016 g C6H12O6Mass percent of C H and OMass C 6 mol C x 1201 gmol C x 100 4000 mass C18016 g C6H. How many moles are in sugar. Molar mass of Hydrogen H1gmol1.
This site is an open community for users to share their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site helpful, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title how many grams of carbon are in 1 mole of glucose c6h12o6 by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






