Your How many grams is one molecule of ethanol images are ready in this website. How many grams is one molecule of ethanol are a topic that is being searched for and liked by netizens now. You can Find and Download the How many grams is one molecule of ethanol files here. Download all free photos and vectors.
If you’re searching for how many grams is one molecule of ethanol images information connected with to the how many grams is one molecule of ethanol interest, you have pay a visit to the ideal site. Our website always provides you with hints for downloading the maximum quality video and image content, please kindly surf and find more informative video articles and graphics that fit your interests.
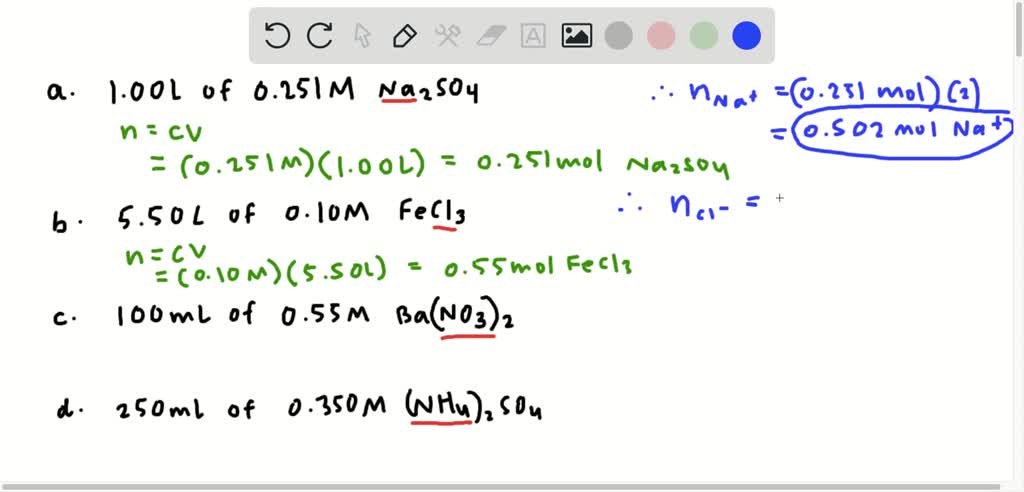
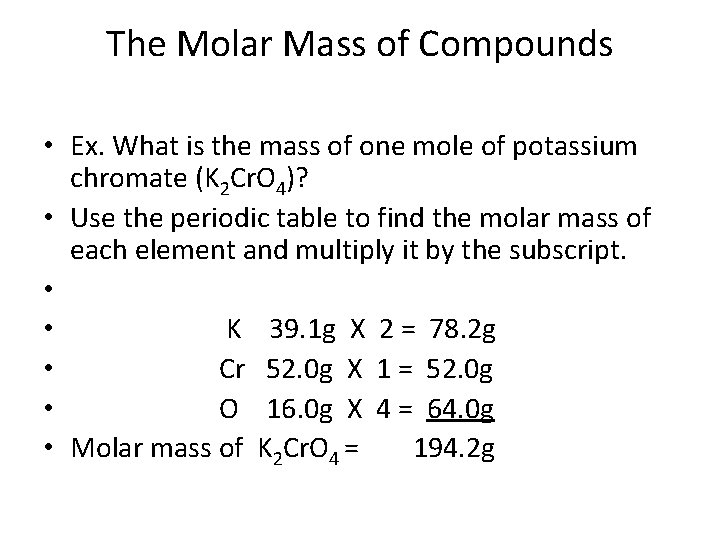
How Many Grams Is One Molecule Of Ethanol. How heavy in grams is one molecule of ethanol. In this case ethanols molecular mass is 46 gmol. It is made of nine atoms that include two carbon C atoms six hydrogen H atoms and one oxygen O atom. We assume you are converting between grams Ethanol and mole.

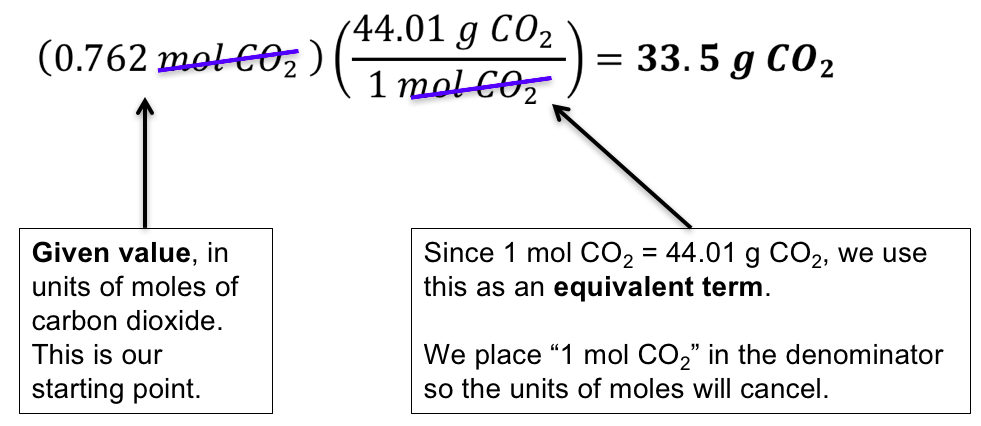
The answer is 4606844. A mole is a unit of many particles atoms molecules ions where 1 mole is the number of particles contained in a substance that is the same amount as many atoms in 12 gr C-12. As one mole is in the SI system refers to 60221411023 units 01982 moles is equal to 11931023 units. The same is true of a mole. In this case ethanols molecular mass is 46 gmol. 167 Based on your answer to CTQ 9 calculate the mass in grams of one mole of methane molecules to 001 g.
The mass of one ethanol C2H6O molecule is 765 10-23 grams.
Click to see full answer. As one mole is in the SI system refers to 60221411023 units 01982 moles is equal to 11931023 units. Bob is working as a Baker in your bakeshop. 1 grams Ethanol is equal to 0. 120107 1007943 120107 1007942 159994 100794 Percent composition by element. The answer is 4606844.
 Source: slidetodoc.com
Source: slidetodoc.com
Calculate the average mass in amu of one methane molecule to 001 amu. In each ethanol molecule there are 2 C 1 O and 6 H atoms 2 x 120108 gmol 159994 gmol 6 x 10079 gmol 460684 gmol B. A mole is a unit of many particles atoms molecules ions where 1 mole is the number of particles contained in a substance that is the same amount as many atoms in 12 gr C-12. The SI base unit for amount of substance is the mole. Ethanols chemical formula is C2H6O.
 Source: slidetodoc.com
Source: slidetodoc.com
Calculate the average mass in amu of one methane molecule to 001 amu. This chemical formula can also be written as CH3CH2OH or C2H5OH. How many grams Ethanol in 1 mol. As one mole is in the SI system refers to 60221411023 units 01982 moles is equal to 11931023 units. The answer is 4606844.
 Source: slideplayer.com
Source: slideplayer.com
Ethanol molecular weight. 593 moles x 460684 g 1 mole 273. This chemical formula can also be written as CH3CH2OH or C2H5OH. How many grams Ethanol in 1 mol. Likewise people ask what are ethanol atoms.
 Source: toppr.com
Source: toppr.com
How many moles are in 10g of ethanol. You can view more details on each measurement unit. 120107 1007943 120107 1007942 159994 100794 Percent composition by element. The SI base unit for amount of substance is the mole. How many hydrogen atoms are found in one mole of methane molecules.
 Source: chemistry.wustl.edu
Source: chemistry.wustl.edu
1 mole is equal to 1 moles Ethanol or 4606844 grams. 1 grams Ethanol is equal to 0. In each ethanol molecule there are 2 C 1 O and 6 H atoms 2 x 120108 gmol 159994 gmol 6 x 10079 gmol 460684 gmol B. The answer is 4606844. The answer is 4606844.
 Source: slidetodoc.com
Source: slidetodoc.com
This chemical formula can also be written as CH3CH2OH or C2H5OH. Calculate the average mass in amu of one methane molecule to 001 amu. A mole is a unit of many particles atoms molecules ions where 1 mole is the number of particles contained in a substance that is the same amount as many atoms in 12 gr C-12. How many grams are in one mole of ch3ch2oh. You can view more details on each measurement unit.
 Source: slideplayer.com
Source: slideplayer.com
Each mole of ethanol has 2 moles of carbon atoms. Bob is working as a Baker in your bakeshop. 1 grams Ethanol is equal to 0. Convert grams Ethanol to moles or moles Ethanol to grams. Use a grammatically correct English sentence to describe how the mass in amu of one molecule.
 Source: chemistry.wustl.edu
Source: chemistry.wustl.edu
Answer 1 of 2. How heavy in grams is one molecule of ethanol. Note that rounding errors may occur so always check the results. Molecular weight of Ethanol or mol The molecular formula for Ethanol is CH3CH2OH. How many hydrogen atoms are found in one mole of methane molecules.
 Source: youtube.com
Source: youtube.com
How many grams Ethanol in 1 mol. We assume you are converting between grams Ethanol and mole. You can view more details on each measurement unit. A mole is a unit of many particles atoms molecules ions where 1 mole is the number of particles contained in a substance that is the same amount as many atoms in 12 gr C-12. The answer is 4606844.
 Source: chemteam.info
Source: chemteam.info
We assume you are converting between grams Ethanol and mole. The answer is 4606844. 1 mole is equal to 1 moles Ethanol or 4606844 grams. Convert grams Ethanol to moles or moles Ethanol to grams. How many moles are in 10g of ethanol.
 Source: blendspace.com
Source: blendspace.com
1 grams Ethanol is equal to 0. A mole is a unit of many particles atoms molecules ions where 1 mole is the number of particles contained in a substance that is the same amount as many atoms in 12 gr C-12. The molar mass M of a substance is the mass of one mole of that substance. As one mole is in the SI system refers to 60221411023 units 01982 moles is equal to 11931023 units. There are 7641x10²³ grams in one molecule of ethanol.
 Source: slidetodoc.com
Source: slidetodoc.com
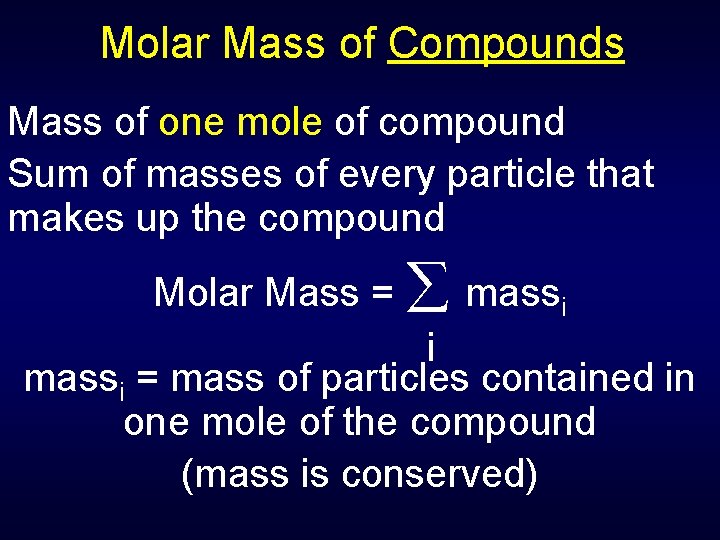
120107 1007943 120107 1007942 159994 100794 Percent composition by element. There are 7641x10²³ grams in one molecule of ethanol. Click to see full answer. Because 1 mol of ethanol contains 2 mol of carbon atoms 2 12011 g 6 mol of hydrogen atoms 6 10079 g and 1 mol of oxygen atoms 1 159994 g its molar mass is 46069 gmol. The molecular mass of a molecule is measured in gmol.

AnswerThe density of ethanol is 0789 gcm³ and there are 1000 cm3 in a liter so 1 liter weighs 0789 kilogramsDensity massvolume So massdensityvolumeSo weight of 1L ethanol 07891. Convert grams Ethanol to moles or moles Ethanol to grams. It has a role as an antiseptic drug a polar solvent a neurotoxin a central nervous system depressant a teratogenic agent a NMDA receptor antagonist a protein kinase C agonist a disinfectant a human metabolite a Saccharomyces cerevisiae metabolite an Escherichia. Molecular weight of Ethanol or mol The molecular formula for Ethanol is CH3CH2OH. The SI base unit for amount of substance is the mole.
 Source: chemistry.wustl.edu
Source: chemistry.wustl.edu
The molar mass M of a substance is the mass of one mole of that substance. The mass of one ethanol C2H6O molecule is 765 10-23 grams. The answer is 4606844. 1 grams Ethanol is equal to 0. Here the number of units is the number of carbon atoms.
 Source: pinterest.com
Source: pinterest.com
Each mole of ethanol has 2 moles of carbon atoms. Molar mass of CH3CH2OH 4606844 gmol. The answer is 4606844. The mass of one ethanol C2H6O molecule is 765 10-23 grams. - 1294502 Case Study Mr.
This site is an open community for users to submit their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site serviceableness, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title how many grams is one molecule of ethanol by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.