Your How many grams is one mole of o2 images are available in this site. How many grams is one mole of o2 are a topic that is being searched for and liked by netizens now. You can Get the How many grams is one mole of o2 files here. Get all royalty-free vectors.
If you’re looking for how many grams is one mole of o2 pictures information connected with to the how many grams is one mole of o2 topic, you have visit the right site. Our site always gives you suggestions for viewing the maximum quality video and picture content, please kindly surf and locate more informative video articles and graphics that fit your interests.
How Many Grams Is One Mole Of O2. Here we are given with 16 g of oxygen. Here the molar mass of oxygen is M O2 M O 2 and the mass is m. In other words 1 mole of oxygen would contain molecules. Sign In to Writing Essays Science.
 How You Measure How Much Ppt Video Online Download From slideplayer.com
How You Measure How Much Ppt Video Online Download From slideplayer.com
1mol H2O 180g X. 1 mole is equal to 1 moles Oxygen or 159994 grams. Thus the number of molecules in 16 gm or 05 mole of oxygen gas are 05 x 6023 x 1023 3012 x 1023 molecules. Here we are given with 16 g of oxygen. In other words 1 mole of oxygen would contain molecules. 199 mg H2O X.
Note that rounding errors may occur so always check the results.
Answered 3 years ago. How many grams of O 2 must react with excess. Here the molar mass of oxygen is M O2 M O 2 and the mass is m. Note that rounding errors may occur so always check the results. Write the expression for the moles of the oxygen. The mass of a mole of Fe2O3 is 2 x Fe 3 x Oxygen 2 x 55845 3 x 1600 15969 grams 1 mole of Fe2O3 15969 grams Fe2O3.
 Source: slideplayer.com
Source: slideplayer.com
199 mg H2O X. 1mol H2O 180g X. Gen Chem Topic Review. The technique used can be applied to any mole to gram conversion. Sign In to Writing Essays Science.
 Source: slideplayer.com
Source: slideplayer.com
The mass of a mole of Fe2O3 is 2 x Fe 3 x Oxygen 2 x 55845 3 x 1600 15969 grams 1 mole of Fe2O3 15969 grams Fe2O3. Here the molar mass of oxygen is M O2 M O 2 and the mass is m. Likewise people ask how many moles are in 16 grams of oxygen. Since oxygen has an atomic mass of 16 gmole the molar mass of oxygen gas O2 is 2 x 16 gmole 32 gmole. All I am doing is taking the.
 Source: slideplayer.com
Source: slideplayer.com
NOg 12O_2g rarr NO_2g Now the stoichiometry CLEARLY indicates that for each mole each 30g of NOg a half-mole quantity 16g of dioxygen gas reacts to give a 46g mass of NO_2g. Answered 3 years ago. Sign In to Tutor. Here the molar mass of oxygen is M O2 M O 2 and the mass is m. I take it that you can calculate the molecular masses of each reactant and product.
 Source: slideplayer.com
Source: slideplayer.com
How many moles of oxygen o2 are in 500 g of oxygen. In other words 1 mole of oxygen would contain molecules. Note that rounding errors may occur so always check the results. 769 X 10-4 mol 320 g 1 mol 246 X 10-2 g. One mole of atoms of oxygen has a mass of 16 g as 16 is the atomic weight of oxygen and contains 602 X 1023 atoms of oxygen.
 Source: pinterest.com
Source: pinterest.com
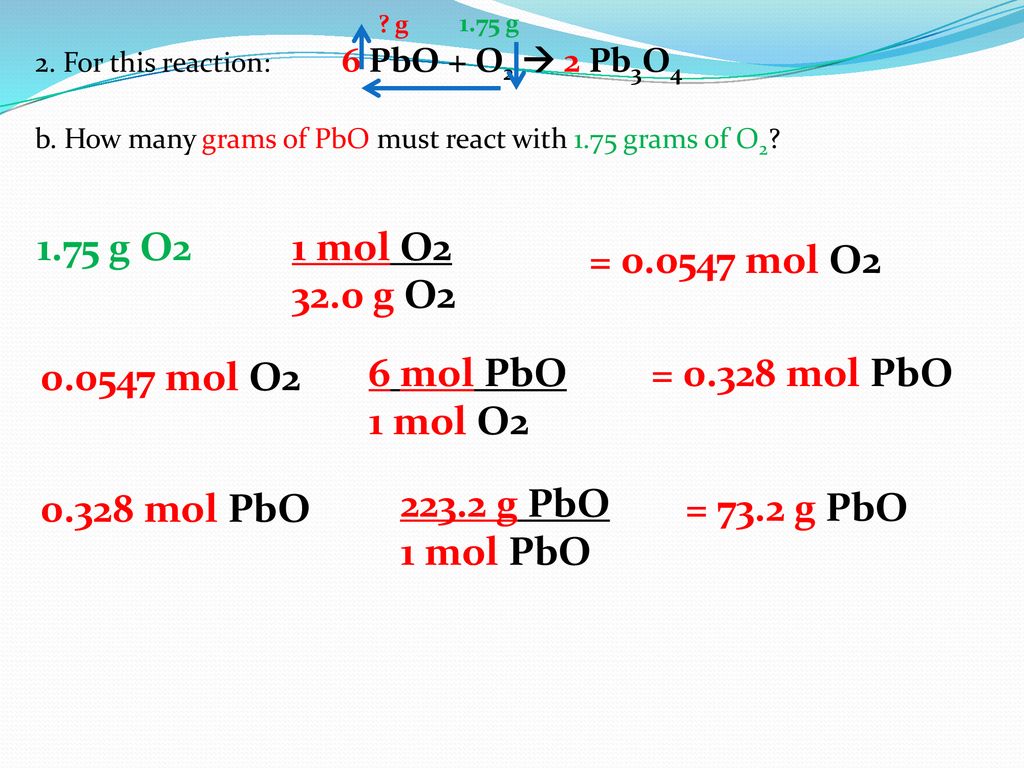
From above balanced equation its clear that we need 5 moles of oxygen to burn 1 mole of C3H8. Since oxygen has an atomic mass of 16 gmole the molar mass of oxygen gas O2 is 2 x 16 gmole 32 gmole. Use this page to learn how to convert between moles Oxygen and gram. The answer is 319988. How many grams of H 2O will form when 109 moles of CH 4 react with excess O 2.
 Source: slideplayer.com
Source: slideplayer.com
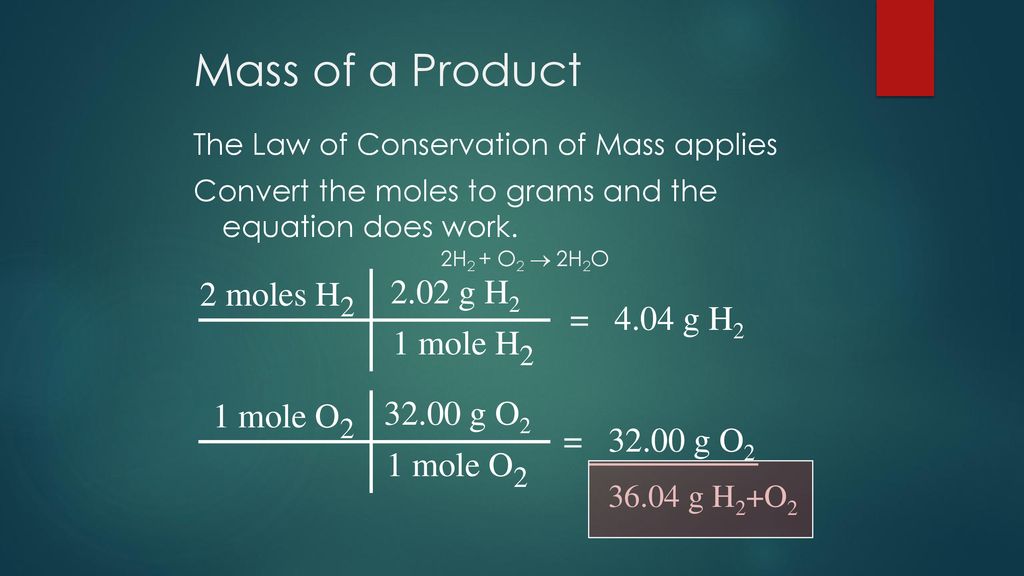
The answer is 319988. Answered 3 years ago. You can view more details on each measurement unit. The molar mass of O2 is 2 x 1600 or 3200 grams 1 mole O2 3200 grams O2. Density of oxygen is equal to 1429 kgm³.
 Source: slideplayer.com
Source: slideplayer.com
Look at your stoichiometry. Here we are given with 16 g of oxygen. In this video well learn to convert moles of O2 to grams. 769 X 10-4 mol 320 g 1 mol 246 X 10-2 g. How many grams of O 2 must react with excess.
 Source: youtube.com
Source: youtube.com
In that way 1 g equivalent of O2 8g. So to burn 100 moles of C3H8 we need 50 moles of oxygen. 1 one mole of O2F2 contains 2 mole of O use. Weight of 1 mole of O2 32g3284 g equivalent. One mole of atoms of oxygen has a mass of 16 g as 16 is the atomic weight of oxygen and contains 602 X 1023 atoms of oxygen.
 Source: youtube.com
Source: youtube.com
In this video well learn to convert moles of O2 to grams. The molar mass of O2 is 2 x 1600 or 3200 grams 1 mole O2 3200 grams O2. General Chemistry Help Homepage. Since 1 mole of oxygen is equivalent to 32 g 4 moles of oxygen gas would be equivalent to 4 moles x 32 gmole 128 g. 1 mole is equal to 1 moles Oxygen or 159994 grams.
 Source: slideplayer.com
Source: slideplayer.com
2 130 moles O. The number of molecules in 16 gm of oxygen are 05 1632 moles. Use this page to learn how to convert between moles O2 and gram. We assume you are converting between grams O2 and mole. So to burn 100 moles of C3H8 we need 50 moles of oxygen.
 Source: slideplayer.com
Source: slideplayer.com
In that way 1 g equivalent of O2 8g. The technique used can be applied to any mole to gram conversion. From above balanced equation its clear that we need 5 moles of oxygen to burn 1 mole of C3H8. We assume you are converting between grams O2 and mole. Oxygen weighs 0001429 gram per cubic centimeter or 1429 kilogram per cubic meter ie.
 Source: pinterest.com
Source: pinterest.com
Since 1 mole of oxygen is equivalent to 32 g 4 moles of oxygen gas would be equivalent to 4 moles x 32 gmole 128 g. You can view more details on each measurement unit. How many grams of O 2 must react with excess. 769 X 10-4 mol 320 g 1 mol 246 X 10-2 g. For our practice problem well.
 Source: youtube.com
Source: youtube.com
NOg 12O_2g rarr NO_2g Now the stoichiometry CLEARLY indicates that for each mole each 30g of NOg a half-mole quantity 16g of dioxygen gas reacts to give a 46g mass of NO_2g. The technique used can be applied to any mole to gram conversion. In that way 1 g equivalent of O2 8g. Since 1 mole of oxygen is equivalent to 32 g 4 moles of oxygen gas would be equivalent to 4 moles x 32 gmole 128 g. G N 2 947 g H 2 x 1 mol H 2 x 1 mol N 2 x 2801g N 2 2016 g H 2 3 mol H 2 1 molN2 439 g N 2 Stoichiometry CH 4 2 O 2 CO 2 2 H 2O Answer the following questions.
 Source: youtube.com
Source: youtube.com
I take it that you can calculate the molecular masses of each reactant and product. Note that rounding errors may occur so always check the results. So to burn 100 moles of C3H8 we need 50 moles of oxygen. Since 1 mole of oxygen is equivalent to 32 g 4 moles of oxygen gas would be equivalent to 4 moles x 32 gmole 128 g. Beside this how many gram atoms are present in 256g of o2.
 Source: youtube.com
Source: youtube.com
The number of molecules in 16 gm of oxygen are 05 1632 moles. How many grams of H 2O will form when 109 moles of CH 4 react with excess O 2. Here the molar mass of oxygen is M O2 M O 2 and the mass is m. Oxygen weighs 0001429 gram per cubic centimeter or 1429 kilogram per cubic meter ie. Thus the number of molecules in 16 gm or 05 mole of oxygen gas are 05 x 6023 x 1023 3012 x 1023 molecules.
This site is an open community for users to do submittion their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site value, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title how many grams is one mole of o2 by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.