Your How many grams is one mole of na images are available. How many grams is one mole of na are a topic that is being searched for and liked by netizens now. You can Get the How many grams is one mole of na files here. Find and Download all free photos and vectors.
If you’re looking for how many grams is one mole of na pictures information related to the how many grams is one mole of na keyword, you have come to the ideal site. Our website frequently gives you hints for refferencing the maximum quality video and image content, please kindly hunt and locate more informative video content and images that fit your interests.
How Many Grams Is One Mole Of Na. C One mole of a monatomic element has a mass equal to its atomic mass expressed in grams. D One mole of water contains 12 mole of oxygen atoms. Remember 1 mol of an ideal gas has a volume of 224 L at STP. 35 grams Na 1 mole Na2299 grams6022 X 10231 mole Na 92 X 1022 atoms of sodium.
 Section 3 6counting Molecules Objectives Define A Mole From slidetodoc.com
Section 3 6counting Molecules Objectives Define A Mole From slidetodoc.com
The molar mass of C is 12011 gramsmol. One mole is in the question How many moles of Na contain 145x10 21 atoms of Na. The molar mass of H is 10079 gramsmol. Multiply the molar mass of sodium by 2 and add sulfurs molar mass. We have 3 oxygens in our chemical formula so well need to multiply that last number by 3. The technique used can be applied to any mole to gram conversion.
If you had 20g of NaCl what percent of the weight is due to the presence of sodium.
Do a quick conversion. We have 3 oxygens in our chemical formula so well need to multiply that last number by 3. 1 mole of copper. One atom of sulfur has a mass of 3207 amu. The SI base unit for amount of substance is the mole. The symbol of mole is mol.
 Source: pinterest.com
Source: pinterest.com
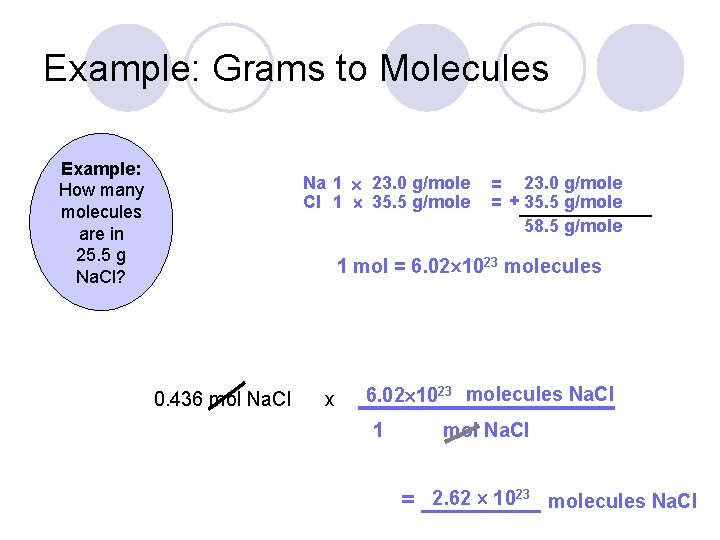
Therefore grams 1 mole times atomic weight of sodium atom. Rounding to one decimal place the mass of one mole of sodium Na is 230 grams and that of chlorine Cl is 355 grams so one mole of NaCl has a mass of 585 grams. 1 grams Na 0043497607849056 mole using the molecular weight calculator and the molar mass of Na. Note that rounding errors may occur so always check the results. 60231023 atoms of Na 1 mol of Na.
 Source: slideplayer.com
Source: slideplayer.com
What is the formula for sodium phosphate. The technique used can be applied to any mole to gram conversion. The SI base unit for amount of substance is the mole. One mole of. How many grams of Na solid are required to completely react with 192 Lof Cl gas at STP according to the following chemical reaction.
 Source: gr.pinterest.com
Source: gr.pinterest.com
E none of the above. 60231023 atoms of Na 1 mol of Na. Rounding to one decimal place the mass of one mole of sodium Na is 230 grams and that of chlorine Cl is 355 grams so one mole of NaCl has a mass of 585 grams. Do a quick conversion. The technique used can be applied to any mole to gram conversion.
 Source: pinterest.com
Source: pinterest.com
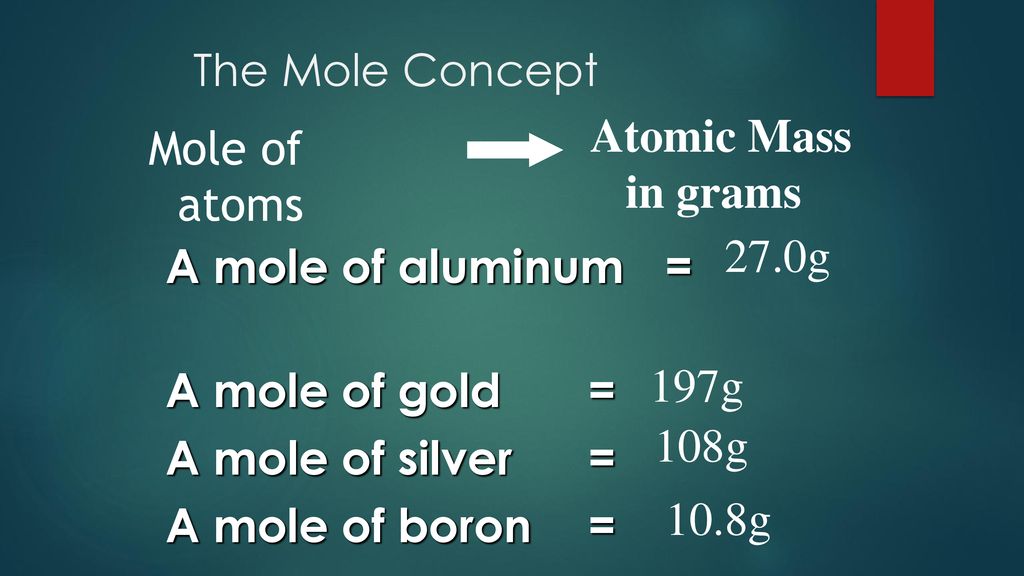
Note that rounding errors may occur so always check the results. The molar mass of Na is 22990 gramsmol. The SI base unit for amount of substance is the mole. A One mole of atoms makes up an amount of atoms that can be seen with the naked eye. 1 mole of Na weighs 16g.
 Source: slideplayer.com
Source: slideplayer.com
1008 amu x 1661 x10-24 gamu 1674 x10-24 g. E none of the above. 1 mole or 602 x 1023 molecules of sodium carbonate Na2CO3 weighs 1060 grams and a scientist could indeed measure 1060 grams using a lab balance. Thus 23 grams are contained in 1 mole of. There are ______ number of molecules atoms of NaCl in one mole of NaCl.
 Source: slidetodoc.com
Source: slidetodoc.com
Thus 23 grams are contained in 1 mole of. Mass of 1 H atom. One mole of. One mole is in the question How many moles of Na contain 145x10 21 atoms of Na. Now you can do some weight percentage problems like.
 Source: youtube.com
Source: youtube.com
In this example multiply the grams of Na by the conversion factor 1 mol Na 2298 g Na with 2298g being the molar mass of one mole of Na which then allows cancelation of grams leaving moles of Na. 1 grams Na 0043497607849056 mole using the molecular weight calculator and the molar mass of Na. One mole of S atoms has a mass of 3207 g. Isnt avagadros number fun. Mass of 1 H atom.
 Source: youtube.com
Source: youtube.com
To find moles divide atoms by Avogadros number 511x10 23 atoms of lithium is equal to how many moles. Do a quick conversion. You have 12 grams which equals 12585 021 moles. 1 mole is equal to 1 moles Na3PO4 or 163940671 grams. For our practice problem well co.
 Source: pinterest.com
Source: pinterest.com
Divide the mass of the sample by the molar mass to obtain moles. A One mole of atoms makes up an amount of atoms that can be seen with the naked eye. In this video well learn to convert moles of Na to grams. One mole of. How many moles of copper are.
 Source: co.pinterest.com
Source: co.pinterest.com
1 mole of Na weighs 16g. Note that rounding errors may occur so always check the results. Divide by molar mass of NaCl which is 5844 gmol NaCl. 2 229897693 gmol 3207 gmol780495386 gmol. One mole of.
 Source: slidetodoc.com
Source: slidetodoc.com
For our practice problem well co. How many grams of Na solid are required to completely react with 192 Lof Cl gas at STP according to the following chemical reaction. The molar mass of C is 12011 gramsmol. Isnt avagadros number fun. What is the formula for sodium phosphate.
 Source: pinterest.com
Source: pinterest.com
1 grams Na 0043497607849056 mole using the molecular weight calculator and the molar mass of Na. 2 23 32 4 16 142 grams per mole. 1 mole is equal to 1 moles KMnO4 or 158033949 grams. One mole is in the question How many moles of Na contain 145x10 21 atoms of Na. Mass of 1 H atom.
 Source: tr.pinterest.com
Source: tr.pinterest.com
The molar mass of H is 10079 gramsmol. One mole of S atoms has a mass of 3207 g. Is Proactiv good for acne. The technique used can be applied to any mole to gram conversion. Mass of 1 H atom.
 Source: pinterest.com
Source: pinterest.com
In this example multiply the grams of Na by the conversion factor 1 mol Na 2298 g Na with 2298g being the molar mass of one mole of Na which then allows cancelation of grams leaving moles of Na. How do you find moles of Na. 142g 142gmol1 01 mol. You have 12 grams which equals 12585 021 moles. Mass of a mole of particles mass of 1 particle x 6022 x 1023 The mass of an atom in amu is numerically the same as the mass of one mole of atoms of the element in grams.
 Source: youtube.com
Source: youtube.com
How many grams of Na solid are required to completely react with 192 Lof Cl gas at STP according to the following chemical reaction. Note that rounding errors may occur so always check the results. The molar mass of Na is 22990 gramsmol. To find moles divide atoms by Avogadros number 511x10 23 atoms of lithium is equal to how many moles. The SI base unit for amount of substance is the mole.
This site is an open community for users to do sharing their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site helpful, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title how many grams is one mole of na by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






