Your How many grams is one mole of n2 images are available in this site. How many grams is one mole of n2 are a topic that is being searched for and liked by netizens now. You can Download the How many grams is one mole of n2 files here. Find and Download all royalty-free images.
If you’re looking for how many grams is one mole of n2 images information connected with to the how many grams is one mole of n2 topic, you have visit the ideal blog. Our site always provides you with suggestions for seeing the highest quality video and picture content, please kindly surf and locate more informative video articles and graphics that fit your interests.
How Many Grams Is One Mole Of N2. So take the molecular mass of elemental oxygen - 16 grams per mole. Note that rounding errors may occur so always check the results. Since M is a fraction you can divide by multiplying by its reciprocal. Find the mass in 26 mol of lithium bromide.
 Quick Review The Mole A Very Large Counting From slidetodoc.com
Quick Review The Mole A Very Large Counting From slidetodoc.com
32 gmol and the. How many grams of O 2 must react with excess. The SI base unit for amount of substance is the mole. LiBr 226 g LiBr 1 mole LiBr. 26 mole LiBr x. On the basis of the given data the molar mass of nitrogen is 2802 g mol the moles of nitrogen given is 460 mol.
435 1345 Views.
26 mole LiBr x. The Molar Mass Of Nitrogen N2 Is 2802 GMol. Note that rounding errors may occur so always check the results. In this manner we can cancel the unit grams and be left with the unit moles in the numerator. Hence there is one mole of N2 in 28 grams of nitrogen N2. One Mole Of N2 Is Combined With 3 Moles Of H2 In Litre If i ask what number of grams Are in a mole of N2 it will be 280134 grams and your asking 19 so 280134 x 19 can be 5322546 grams.
 Source: slidetodoc.com
Source: slidetodoc.com
Note that rounding errors may occur so always check the results. How many grams of O 2 must react with excess. LiBr 226 g LiBr 1 mole LiBr. Find the grams in 126 x 10-4 mol of HC2H3O2. 26 mole LiBr x.
 Source: youtube.com
Source: youtube.com
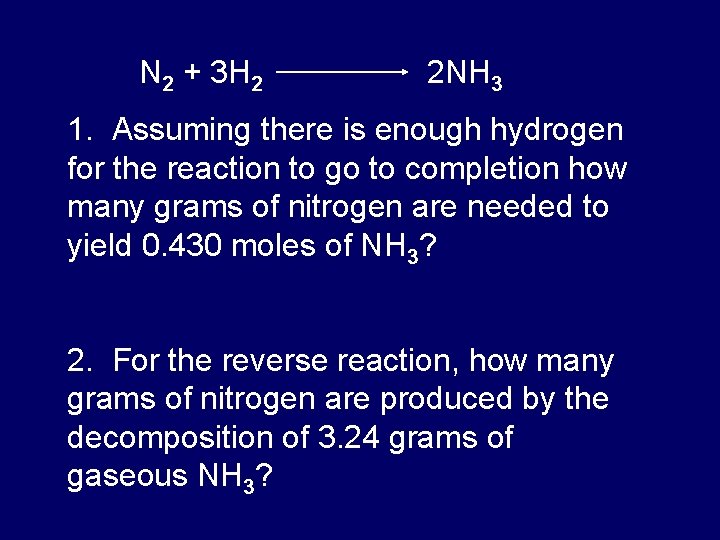
1 mole is equal to 1 moles NH3 or 1703052 grams. 340 In the Haber process ammonia NH3 is manufactured from nitrogen N2 and hydrogen H2 according to the reaction N2 3H2 – 2NH3- Use this equation to answer this question. How much does nitrogen gas weigh in grams. Answer to How many grams of N2 are needed to produce 217 mol of NH3 when reacted according to this chemical equationN2g 3H2g 2NH3g SolutionInn. The SI base unit for amount of substance is the mole.
 Source: gr.pinterest.com
Source: gr.pinterest.com
The SI base unit for amount of substance is the mole. 1 mol N228014 g N2 004 mol N2 to 1 vital determine. The SI base unit for amount of substance is the mole. Molecular weight of NH3 or grams This compound is also known as Ammonia. For our practice problem well co.
 Source: slidetodoc.com
Source: slidetodoc.com
You can view more details on each measurement unit. 1 mole is equal to 1 moles N2 or 280134 grams. You can view more details on each measurement. Since the molar mass of nitrogen atomic nitrogen is 1401g we simply double the number. 2 How many moles of N2 are in 1L.
 Source: youtube.com
Source: youtube.com
4 rows How much does 1 mole of N2 weigh in Gram. In 2 moles of nitric acid there are 2 moles of hydrogen atoms 6 moles of oxygen atoms and 2 moles of nitrogen atoms. How many grams is a mole of N2. Answer to How many grams of N2 are needed to produce 217 mol of NH3 when reacted according to this chemical equationN2g 3H2g 2NH3g SolutionInn. So in one mole of nitrogen gas you have 6022 1023 molecules of nitrogen gas N2.
 Source: youtube.com
Source: youtube.com
In 2 moles of nitric acid there are 2 moles of hydrogen atoms 6 moles of oxygen atoms and 2 moles of nitrogen atoms. What number of grams is 1 mole of n2. Hence there is one mole of N2 in 28 grams of nitrogen N2. How many moles is 28 grams of n. So take the molecular mass of elemental oxygen - 16 grams per mole.
 Source: pinterest.com
Source: pinterest.com
On the basis of the given data the molar mass of nitrogen is 2802 g mol the moles of nitrogen given is 460 mol. Since the molar mass of nitrogen atomic nitrogen is 1401g we simply double the number. One mole equals 6 x 1023 atomsmoleculeswhatever. The Molar Mass Of Nitrogen N2 Is 2802 GMol. 32 gmol and the.

Since the molar mass of nitrogen atomic nitrogen is 1401g we simply double the number. Type in your own numbers in the form to convert the units. The Molar Mass Of Nitrogen N2 Is 2802 GMol. One mole equals 6 x 1023 atomsmoleculeswhatever. N mM where n moles m mass in grams and M molar mass in gmol.
 Source: slideplayer.com
Source: slideplayer.com
Alternatively you can express this as 2 N A where N A is Avogadros constant. Find the grams in 126 x 10-4 mol of HC2H3O2. Molar mass N 2 N 2 - 2802 gmol. Answer to How many grams of N2 are needed to produce 217 mol of NH3 when reacted according to this chemical equationN2g 3H2g 2NH3g SolutionInn. One mole equals 6 x 1023 atomsmoleculeswhatever.
 Source: slidetodoc.com
Source: slidetodoc.com
We assume you are converting between moles NH3 and gram. On the basis of the given data the molar mass of nitrogen is 2802 g mol the moles of nitrogen given is 460 mol. You can view more details on each measurement. So in one mole of nitrogen gas you have 6022 1023 molecules of nitrogen gas N2. The mass of given amount of nitrogen atoms is 70 grams.
 Source: khanacademy.org
Source: khanacademy.org
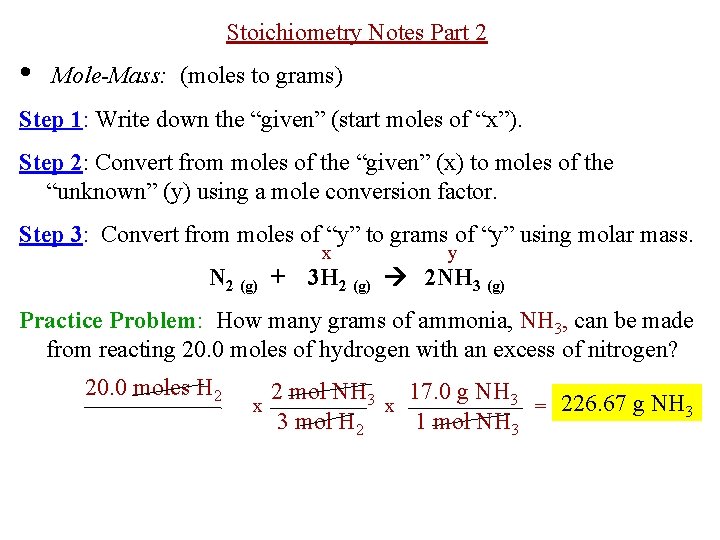
How many grams is a mole of N2. 32 gmol and the. Molecular weight of NH3 or grams This compound is also known as Ammonia. Moles N2 344 g. The technique used can be applied to any mole to gram conversion.
 Source: gr.pinterest.com
Source: gr.pinterest.com
6006 g HC2H3O2 000767 g HC2H3O2 1 mole HC2H3O2. 340 In the Haber process ammonia NH3 is manufactured from nitrogen N2 and hydrogen H2 according to the reaction N2 3H2 – 2NH3- Use this equation to answer this question. 129 grams The correct answer is 129 grams. The mass of given amount of nitrogen atoms is 70 grams. 1 mole elemental oxygen16 grams O1 mole 16 grams.
 Source: youtube.com
Source: youtube.com
Note that rounding errors may occur so always check the results. Click to see full answer. The molar mass M of N2 is 28014 gmol. On the basis of the given data the molar mass of nitrogen is 2802 g mol the moles of nitrogen given is 460 mol. What is the mass of 5 moles of nitrogen.
 Source: slideplayer.com
Source: slideplayer.com
We assume you are converting between moles NH3 and gram. What number of grams is 1 mole of n2. 1 mole is equal to 1 moles N2 or 280134 grams. The SI base unit for amount of substance is the mole. 340 In the Haber process ammonia NH3 is manufactured from nitrogen N2 and hydrogen H2 according to the reaction N2 3H2 – 2NH3- Use this equation to answer this question.
 Source: youtube.com
Source: youtube.com
G N 2 947 g H 2 x 1 mol H 2 x 1 mol N 2 x 2801g N 2 2016 g H 2 3 mol H 2 1 molN2 439 g N 2 Stoichiometry CH 4 2 O 2 CO 2 2 H 2O Answer the following questions. Note that rounding errors may occur so always check the results. We assume you are converting between moles NH3 and gram. How many moles of O 2 are required to react with 172 moles of CH 4. Click to see full answer.
This site is an open community for users to submit their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site value, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title how many grams is one mole of n2 by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






