Your How many grams is one mole of ki images are ready. How many grams is one mole of ki are a topic that is being searched for and liked by netizens today. You can Find and Download the How many grams is one mole of ki files here. Download all royalty-free photos and vectors.
If you’re looking for how many grams is one mole of ki pictures information connected with to the how many grams is one mole of ki interest, you have come to the right site. Our site always gives you hints for seeing the maximum quality video and picture content, please kindly search and locate more enlightening video articles and images that fit your interests.
How Many Grams Is One Mole Of Ki. 133 What is the number of grams of unknown soluteMW 56105 in 250 mL of a 0169M solution. The SI base unit for amount of substance is the mole. You can view more details on each measurement unit. In the reaction Zn H2SO4 ZnSO4 H2 how many grams of H2SO4 are required to produce 10 gram of H2.
 If One Mole Of Carbon Atoms Weighs 12 Grams What Is The Mass In Grams Of 1 Atom Of Carbon Youtube From youtube.com
If One Mole Of Carbon Atoms Weighs 12 Grams What Is The Mass In Grams Of 1 Atom Of Carbon Youtube From youtube.com
Putting values in equation 1 we get. Note that rounding errors may occur so always check the results. The molar mass of KI is 166003 gramsmole. The answer is 46100894. By the stoichiometry of the reaction there will be half an equiv of lead iodide per equiv of potassium iodide. 1 mole is equal to 1 moles KMnO4 or 158033949 grams.
To make a 1 M solution of KCl you would dissolve 740 grams in one liter of water.
How many moles are present in 10 grams of sodium hydroxide. The answer is rounded to three sig figs the number of sig figs you have for the molarity of the solution. How many moles are present in 10 grams of sodium hydroxide. For 1 liter of a 320 M solution we need 320 moles of solute. KI mole weight is 390983 12690 1659983 gm. The chemical equation for the reaction of potassium iodide.
 Source: youtube.com
Source: youtube.com
By the stoichiometry of the reaction there will be half an equiv of lead iodide per equiv of potassium iodide. Type in your own numbers in the form to convert the units. How many grams is Fe2O3. 0100 mole x 166003 gramsmole 166 gram when rounded off to the correct number of significant figures Set up as a proportion. 1 mole is equal to 1 moles Fe2O3 or 1596882 grams.
 Source: youtube.com
Source: youtube.com
Note that rounding errors may occur so always check the results. How many grams is Fe2O3. The SI base unit for amount of substance is the mole. Answer 1 of 5. The answer is 46100894.
 Source: youtube.com
Source: youtube.com
The answer is rounded to three sig figs the number of sig figs you have for the molarity of the solution. 1 Given mass of lead II iodide 12 g. How many moles of FeCl2 are prduced. 0100 L x 0300 molL x 16600277 g KImol 498 g KI. Use this page to learn how to convert between grams KI and mole.

1 mole is equal to 1 moles Potassium Iodide or 16600277 grams. Molecular weight of PbI2 or grams This compound is also known as LeadII Iodide. 0825moles KI 166003 g 1mole KI 137 g. 1 mole is equal to 1 moles Potassium Iodide or 16600277 grams. How many grams is Fe2O3.
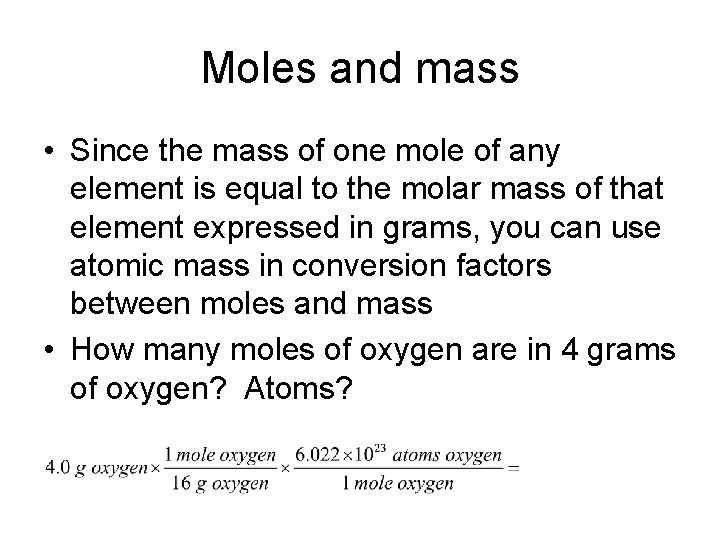 Source: slidetodoc.com
Source: slidetodoc.com
From the equation TWO moles of KI will result in ONE mole PbI2. This can be done using the molecular weight of KHP. To make a 1 M solution of KCl you would dissolve 740 grams in one liter of water. The answer is 00021691553313478. 0100 L x 0300 molL x 16600277 g KImol 498 g KI.
 Source: youtube.com
Source: youtube.com
0056 kilograms in 1 mole of iron. The answer is 3999711. Grams to moles Molar mass N 2O. Type in your own numbers in the form to convert the units. 1 mole is equal to 1 moles KI or 16600277 grams.
 Source: youtube.com
Source: youtube.com
The molar mass of KI is 166003 gramsmole. 0100 L x 0300 molL x 16600277 g KImol 498 g KI. Note that rounding errors may occur so always check the results. First you must calculate the number of moles in this solution by rearranging the equation. This compound is also known as Lead II Iodide.
 Source: courses.lumenlearning.com
Source: courses.lumenlearning.com
Note that rounding errors may occur so always check the results. By the stoichiometry of the reaction there will be half an equiv of lead iodide per equiv of potassium iodide. The answer is rounded to three sig figs the number of sig figs you have for the molarity of the solution. How many grams are in 150 moles of KMnO4. Moles of mol KI -12mol PbI_2s You have done the hard yakka in quoting the balanced chemical equation.
 Source: slideplayer.com
Source: slideplayer.com
Its easier to work with grams so convert the mass. We assume you are converting between grams PbI2 and mole. Note that rounding errors may occur so always check the results. Given the reaction Zn 2HCl ZnCl2 H2. Note that rounding errors may occur so always check the results.
 Source: slidetodoc.com
Source: slidetodoc.com
How many grams are in 150 moles of KMnO4. 0100 mole x 166003 gramsmole 166 gram when rounded off to the correct number of significant figures Set up as a proportion. 1 Given mass of lead II iodide 12 g. 1 mole is equal to 1 moles Fe2O3 or 1596882 grams. 1 mole is equal to 1 moles Potassium Iodide or 16600277 grams.
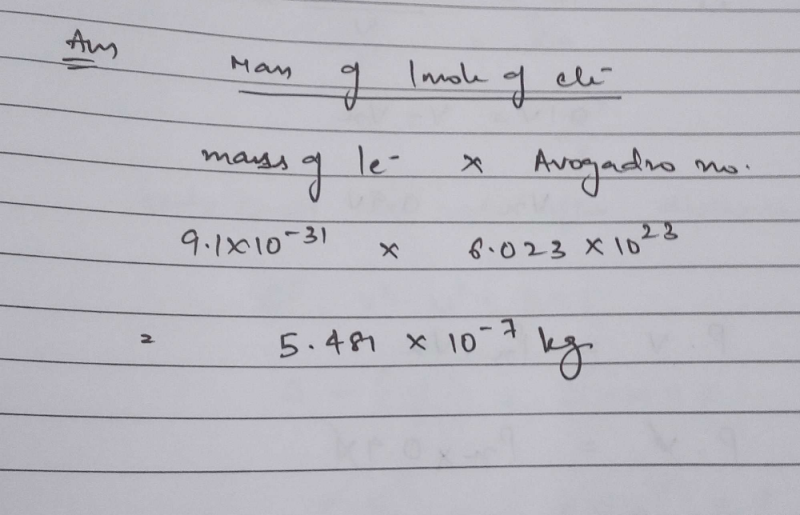 Source: edurev.in
Source: edurev.in
5988 kg 5988 g. The answer is rounded to three sig figs the number of sig figs you have for the molarity of the solution. Putting values in equation 1 we get. Hope this makes sense. You can view more details on each measurement unit.
 Source: youtube.com
Source: youtube.com
How many milliliters of 1500M aqueous KI solution must be added to an aqueous solution containing 100 mole of PbNO32 to completely precipitate the lead. This compound is also known as Lead II Iodide. We assume you are converting between moles PbI2 and gram. We assume you are converting between grams NaOH and mole. N 5988 g 18015 gmol 3324 mol.
 Source: chegg.com
Source: chegg.com
Note that rounding errors may occur so always check the results. How many moles PbI2 in 1 grams. You can always use our grams to moles calculator to check the result. How many moles of FeCl2 are prduced. The SI base unit for amount of substance is the mole.
 Source: youtube.com
Source: youtube.com
What is the molar mass of KI. For 1 liter of a 320 M solution we need 320 moles of solute. How many kilograms are there in 1 mole of iron. 133 What is the number of grams of unknown soluteMW 56105 in 250 mL of a 0169M solution. From the equation TWO moles of KI will result in ONE mole PbI2.
 Source: youtube.com
Source: youtube.com
After converting to moles I simply used the molar mass of KI obtained from your periodic table to covert from moles to grams. This compound is also known as Lead II Iodide. How many moles are present in 10 grams of sodium hydroxide. To make a 1 M solution of KCl you would dissolve 740 grams in one liter of water. The SI base unit for amount of substance is the mole.
This site is an open community for users to submit their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site value, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title how many grams is one mole of ki by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






