Your How many grams is one mole of hydrogen gas images are ready in this website. How many grams is one mole of hydrogen gas are a topic that is being searched for and liked by netizens today. You can Find and Download the How many grams is one mole of hydrogen gas files here. Get all free images.
If you’re searching for how many grams is one mole of hydrogen gas pictures information linked to the how many grams is one mole of hydrogen gas keyword, you have pay a visit to the ideal site. Our website always gives you hints for viewing the maximum quality video and picture content, please kindly hunt and locate more enlightening video articles and graphics that fit your interests.
How Many Grams Is One Mole Of Hydrogen Gas. Hydrogen weighs 0000082 gram per cubic centimeter or 0082 kilogram per cubic meter ie. These numbers are either in atomic mass units amu or in grams per mole of atoms. Contains two atoms of hydrogen. It depends on the substance.
 Ideal Gas Law 1 Avogadros Principle N How From slidetodoc.com
Ideal Gas Law 1 Avogadros Principle N How From slidetodoc.com
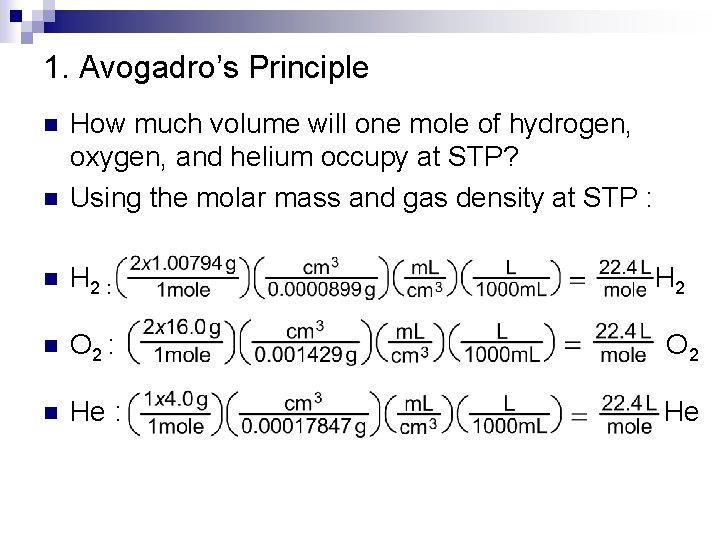
1008 gramsmole Hydrogen 1 mole6022x1023 atoms 167 x 10-24 grams. After 5 minutes 198 10-3 moles of hydrogen gas is produced. In the example the amount of hydrogen is 202650 x 0025 29315 x 8314472 2078 moles. If one mole of hydrogen is equal to 101 grams then does one mole dihydrogen equal 202 grams. Note that rounding errors may occur so always check the results. What is the total mass of 20 moles of hydrogen gas.
Use this page to learn how to convert between grams Hydrogen and mole.
One mole of zinc will react with 2 moles of aqueous hydrogen chloride to form one mole of zinc chloride and one mole of hydrogen gas. Moles of H2 25mol Sn 1 mol H2 1mol Sn 25 mol H2. 14 grams hydrogen 1 mole H1008 grams 1389 moles hydrogen which you can call 14 moles of hydrogen. 1 mole of hydrogen gas contains 60210 23 atoms. Mass of H2 25mol H2 2016 g H2 1mol H2 50 g H2. 1008 gramsmole Hydrogen 1 mole6022x1023 atoms 167 x 10-24 grams.

At 0C 32F or 27315K at standard atmospheric pressure. How many moles in 1 gram. 33 g C3H8 1 mol C3H8 4 mol H2O 1802 g H2O 54 g H2O 4411 g C3H8 1 mol C3H8 1 mol H2O 6 K3PO4 AlNO33 3 KNO3 AlPO4 How many grams of potassium nitrate are produced from 105 grams of aluminum nitrate. Use the mass of the hydrogen gas to calculate the gas moles directly. 1 mole 2 gmol 2 mole 365 gmol 2 g 73 g.
 Source: slideplayer.com
Source: slideplayer.com
1 grams Hydrogen is equal to 0992122546977 mole. 16 How many moles of bromine gas are in 377 grams. 1 mole of M g reacts with 2 moles of H C l and forms 1 mole of hydrogen gas. In the example the amount of hydrogen is 202650 x 0025 29315 x 8314472 2078 moles. At 0C 32F or 27315K at standard atmospheric pressure.
 Source: studylib.net
Source: studylib.net
1 mole of M g reacts with 2 moles of H C l and forms 1 mole of hydrogen gas. If you started this reaction with 65 grams of zinc how many grams of hydrogen would you expect to make. Remember that moles are a. Contains two atoms of hydrogen. 1 molecule of hydrogen H 2.
 Source: slideplayer.com
Source: slideplayer.com
Therefore 2 moles will have a mass of 40 grams. After 5 minutes 198 10-3 moles of hydrogen gas is produced. 33 g C3H8 1 mol C3H8 4 mol H2O 1802 g H2O 54 g H2O 4411 g C3H8 1 mol C3H8 1 mol H2O 6 K3PO4 AlNO33 3 KNO3 AlPO4 How many grams of potassium nitrate are produced from 105 grams of aluminum nitrate. Remember that moles are a. This means that regardless of how many moles of nitrogen gas you have the reaction will always consume twice as many moles of hydrogen gas.
 Source: slideplayer.com
Source: slideplayer.com
You are correct if you thought the answer was one. In the example the amount of hydrogen is 202650 x 0025 29315 x 8314472 2078 moles. A 0236 B 0472 C 3 301 10 D 799 E none of the above. Zn s 2HCl aq ZnCl 2 aq H 2 g Question. We assume you are converting between moles In and gram.
 Source: slidetodoc.com
Source: slidetodoc.com
Note that rounding errors may occur so always check the results. How many moles are in a gram. Hence option B is correct. 1 mole is equal to 1 moles Hydrogen or 100794 grams. What is the total mass of 20 moles of hydrogen gas.
 Source: pinterest.com
Source: pinterest.com
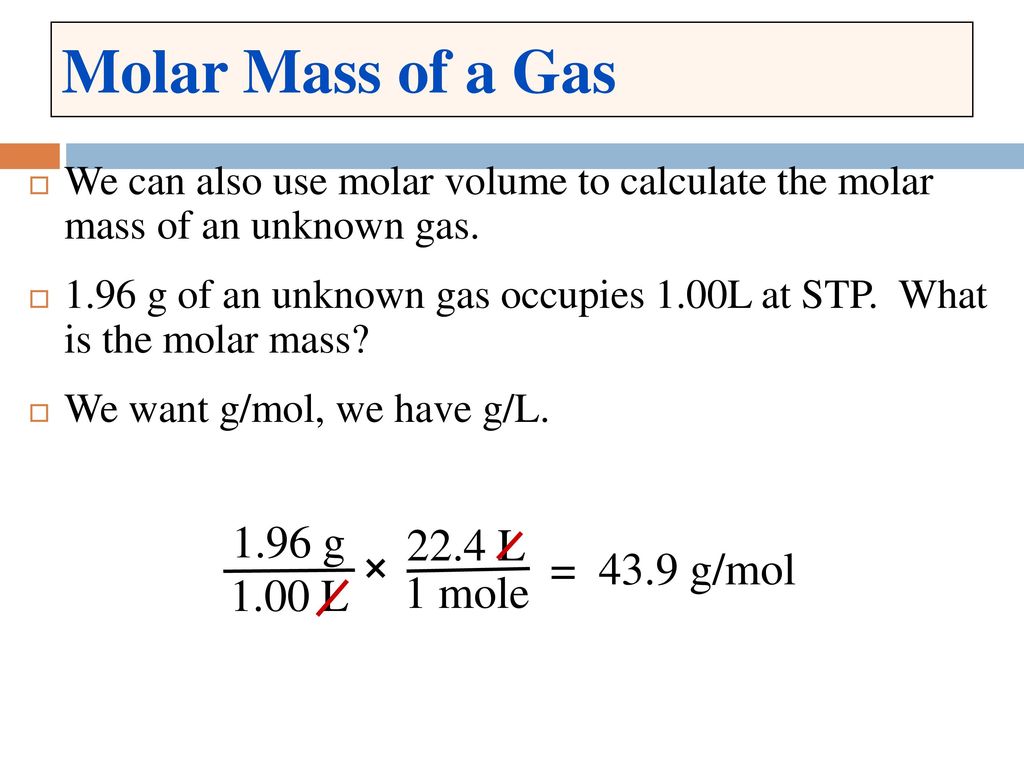
The chemical formula of hydrogen gas is H2 so its molar mass M is 2 gmol-1. 1 mole is equal to 1 moles O2 or 319988 grams. Use this page to learn how to convert between grams Hydrogen and mole. Remember that moles are a. Density of hydrogen is equal to 0082 kgm³.
 Source: slideplayer.com
Source: slideplayer.com
How many moles are in a gram. 1 mole is equal to 1 moles O2 or 319988 grams. A 0236 B 0472 C 3 301 10 D 799 E none of the above. 1 mole 2 gmol 2 mole 365 gmol 2 g 73 g. 1 gm of hydrogen contains 2160210 23.
 Source: slidetodoc.com
Source: slidetodoc.com
1 grams Hydrogen is equal to 0992122546977 mole. You are correct if you thought the answer was one. These numbers are either in atomic mass units amu or in grams per mole of atoms. Hydrogen weighs 0000082 gram per cubic centimeter or 0082 kilogram per cubic meter ie. At 0C 32F or 27315K at standard atmospheric pressure.
 Source: youtube.com
Source: youtube.com
After 5 minutes 198 10-3 moles of hydrogen gas is produced. How many grams are in 722 moles of HF. Zn s 2HCl aq ZnCl 2 aq H 2 g Question. So if you have 2 moles of nitrogen taking part in the reaction you will need. N2 g 3H2 g 2NH3 g Notice that you have a 13 mole ratio between nitrogen gas and hydrogen gas.
 Source: slideplayer.com
Source: slideplayer.com
Solve any question of Some Basic Concepts of Chemistry with-. Solve any question of Some Basic Concepts of Chemistry with-. It depends on the substance. So we come to know that 2 g of hydrogen gives 73 g of hydrochloric acid HCl then how many grams of hydrogen will be required to produce 20 grams of hydrochloric acid HCl. 1 10 -6.
 Source: studylib.net
Source: studylib.net
Based on that information to convert 722 moles of hafnium to grams we multiply 722 moles of hafnium by 17849. Divide the hydrogen weight by its molar mass of 2 gmole. Note that rounding errors may occur so always check the results. We assume you are converting between moles In and gram. 1 10 -6.
 Source: slidetodoc.com
Source: slidetodoc.com
Moles of H2 25mol Sn 1 mol H2 1mol Sn 25 mol H2. Moles H 2 100 g H 2 x 1 mole 495 mol H 2 available 202 g Moles O 2 750 g O 2x 1 mole 234 mol O 2 available 320 g Method 1 Limiting Reactants Chapter 4 moles O 2 needed 495 mol H 2 x 1 mol O 2 2 mol H 2 248 moles O 2 Step 2 Pick H 2 and find the moles O 2 needed to react with all of the H 2 Limiting Reactants Chapter 4 Step 3. Convert from moles to grams 2172 mol AgBr 1878 g AgBr 1 mol AgBr 4079 g AgBr This is the theoretical yield Yields 22. Determine how many moles of AgBr can be formed from this amount of MgBr 2 ie 1086 moles 2 mol AgBr 1 mol MgBr 2 1086 mol MgBr 2 2172 mol AgBr Step 3. 1 mole is equal to 1 moles Hydrogen or 100794 grams.
 Source: slideplayer.com
Source: slideplayer.com
At 0C 32F or 27315K at standard atmospheric pressure. Zn s 2HCl aq ZnCl 2 aq H 2 g Question. N2 g 3H2 g 2NH3 g Notice that you have a 13 mole ratio between nitrogen gas and hydrogen gas. Therefore 2 moles will have a mass of 40 grams. So if you have 2 moles of nitrogen taking part in the reaction you will need.
 Source: pinterest.com
Source: pinterest.com
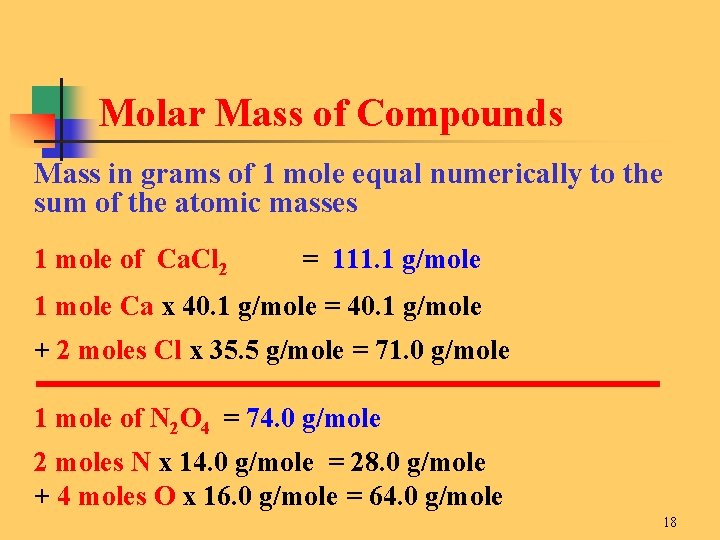
Therefore the option is B. This means that 1 MOLE of hydrogen atoms will weigh 1008 grams. Convert from moles to grams 2172 mol AgBr 1878 g AgBr 1 mol AgBr 4079 g AgBr This is the theoretical yield Yields 22. 1 molecule of hydrogen H 2. Density of hydrogen is equal to 0082 kgm³.
This site is an open community for users to do submittion their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site helpful, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title how many grams is one mole of hydrogen gas by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






